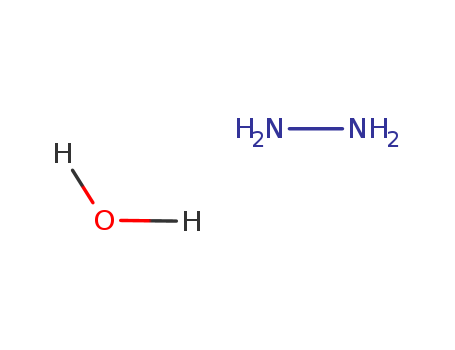
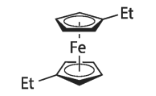
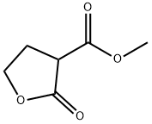
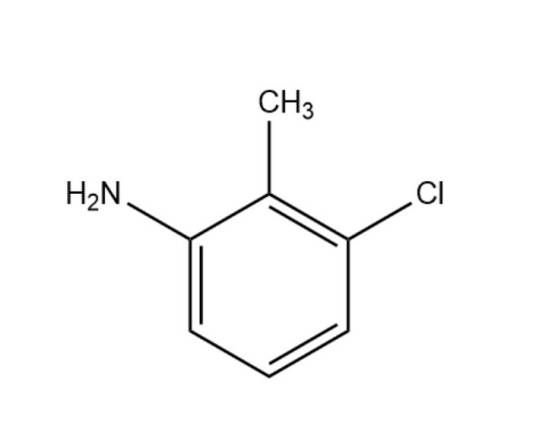
Hydrazine hydrate literature
Boosting Electroreduction Kinetics of Nitrogen to Ammonia via Tuning Electron Distribution of Single-Atomic Iron Sites
Li, Yan,Li, Junwei,Huang, Junheng,Chen, Junxiang,Kong, Yan,Yang, Bin,Li, Zhongjian,Lei, Lecheng,Chai, Guoliang,Wen, Zhenhai,Dai, Liming,Hou, Yang
, p. 9078 - 9085 (2021)
Electrocatalytic nitrogen reduction reaction (NRR) plays a vital role for next-generation electrochemical energy conversion technologies. However, the NRR kinetics is still limited by the sluggish hydrogenation process on noble-metal-free electrocatalyst. Herein, we report the rational design and synthesis of a hybrid catalyst with atomic iron sites anchored on a N,O-doped porous carbon (FeSA-NO-C) matrix of an inverse opal structure, leading to a remarkably high NH3 yield rate of 31.9 μg (Formula presented.) h?1 mg?1cat. and Faradaic efficiency of 11.8 % at ?0.4 V for NRR electrocatalysis, outperformed almost all previously reported atomically dispersed metal-nitrogen-carbon catalysts. Theoretical calculations revealed that the observed high NRR catalytic activity for the FeSA-NO-C catalyst stemmed mainly from the optimized charge-transfer between the adjacent O and Fe atoms homogenously distributed on the porous carbon support, which could not only significantly facilitate the transportation of N2 and ions but also effectively decrease the binding energy between the isolated Fe atom and *N2 intermediate and the thermodynamic Gibbs free energy of the rate-determining step (*N2 → *NNH).
Vacancy Engineering of Iron-Doped W18O49 Nanoreactors for Low-Barrier Electrochemical Nitrogen Reduction
Dou, Shi Xue,Guo, Haipeng,Liang, Ji,Liu, Daolan,Liu, Jian,Lu, Gao Qing,Su, Panpan,Tong, Yueyu,Yan, Xiao,Zhou, Si
supporting information, p. 7356 - 7361 (2020/03/30)
The electrochemical nitrogen reduction reaction (NRR) is a promising energy-efficient and low-emission alternative to the traditional Haber–Bosch process. Usually, the competing hydrogen evolution reaction (HER) and the reaction barrier of ambient electrochemical NRR are significant challenges, making a simultaneous high NH3 formation rate and high Faradic efficiency (FE) difficult. To give effective NRR electrocatalysis and suppressed HER, the surface atomic structure of W18O49, which has exposed active W sites and weak binding for H2, is doped with Fe. A high NH3 formation rate of 24.7 μg h?1 mgcat?1 and a high FE of 20.0 % are achieved at an overpotential of only ?0.15 V versus the reversible hydrogen electrode. Ab initio calculations reveal an intercalation-type doping of Fe atoms in the tunnels of the W18O49 crystal structure, which increases the oxygen vacancies and exposes more W active sites, optimizes the nitrogen adsorption energy, and facilitates the electrocatalytic NRR.
A No-Sweat Strategy for Graphene-Macrocycle Co-assembled Electrocatalyst toward Oxygen Reduction and Ambient Ammonia Synthesis
Biswas, Ashmita,Das, Manisha,Dey, Ramendra Sundar,Kamboj, Navpreet,Sarkar, Subhajit
supporting information, p. 16385 - 16397 (2020/12/03)
Toward the realm of sustainable energy, the development of efficient methods to enhance the performance of electrocatalysts with molecular level perception has gained immense attention. Inspite of untiring attempts, the production cost and scaling-up issues have been a step back toward the commercialization of the electrocatalysts. Herein, we report a one-pot electrophoretic exfoliation technique with minimum time and power input to synthesize iron phthalocyanine functionalized high-quality graphene sheets (G-FePc). The ?-stacked co-assembly excels in oxygen reduction performance (major criterion for fuel cells) with a high positive E1/2 of 0.91 V (vs RHE) and a reproducible reduction peak potential of 0.90 V (vs RHE). An overpotential as low as 29 mV dec-1 and complete tolerance toward the methanol crossover effect confirm the authentication of the catalytic performance of our designed catalyst G-FePc. The catalyst simultaneously exhibits hydrogen storage efficacy by means of nitrogen fixation, yielding 27.74 μg h-1 mgcat-1 NH3 at a potential of-0.3 V (vs RHE) in an acidic electrolyte. The structure-function relationship of the catalyst is revealed via molecular orbital chemistry for the bonding of the Fe(II) active center with O2 and N2 during catalysis.
Oxygen vacancy-engineered Fe2O3 nanocubes: Via a task-specific ionic liquid for electrocatalytic N2 fixation
Zhang, Chenyun,Liu, Shuai,Chen, Tingting,Li, Zhonghao,Hao, Jingcheng
supporting information, p. 7370 - 7373 (2019/06/27)
A task-specific ionic liquid strategy was, for the first time, proposed for designing oxygen vacancy-rich α-Fe2O3 nanocubes toward electrocatalytic N2 fixation to NH3 under ambient conditions, offering a NH3 formation rate of 32.13 μg h-1 mgcat-1 with a faradaic efficiency of 6.63% at -0.3 V vs. the reversible hydrogen electrode.