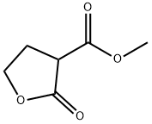
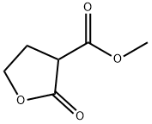
3-FURANCARBOXYLIC ACID, TETRAHYDRO-2-OXO-, METHYL ESTER literature
BICYCLIC HETEROARENES AND METHODS OF THEIR USE
-
Page/Page column 67-68, (2021/12/30)
Disclosed are compounds useful in the treatment of neurological disorders. The compounds described herein, alone or in combination with other pharmaceutically active agents, can be used for treating or preventing neurological diseases.
A Unified Strategy for the Synthesis of Difluoromethyl- And Vinylfluoride-Containing Scaffolds
Duchemin, Nicolas,Buccafusca, Roberto,Daumas, Marc,Ferey, Vincent,Arseniyadis, Stellios
supporting information, p. 8205 - 8210 (2019/10/16)
Here, we report a general method for the synthesis of quaternary and tertiary difluoromethylated compounds and their vinylfluoride analogues. The strategy, which relies on a two-step sequence featuring a C-selective electrophilic difluoromethylation and either a palladium-catalyzed decarboxylative protonation or a Krapcho decarboxylation, is practical, scalable, and high yielding. Considering the generality of the method and the attractive properties offered by the difluoromethyl group, this approach provides a valuable tool for late-stage functionalization and drug development.
Pyrrolidines and Piperidines by Ligand-Enabled Aza-Heck Cyclizations and Cascades of N-(Pentafluorobenzoyloxy)carbamates
Hazelden, Ian R.,Carmona, Rafaela C.,Langer, Thomas,Pringle, Paul G.,Bower, John F.
supporting information, p. 5124 - 5128 (2018/03/26)
Ligand-enabled aza-Heck cyclizations and cascades of N-(pentafluorobenzoyloxy)carbamates are described. These studies encompass the first examples of efficient non-biased 6-exo aza-Heck cyclizations. The methodology provides direct and flexible access to carbamate protected pyrrolidines and piperidines.
Lead optimization of a dihydropyrrolopyrimidine inhibitor against phosphoinositide 3-kinase (PI3K) to improve the phenol glucuronic acid conjugation
Kawada, Hatsuo,Ebiike, Hirosato,Tsukazaki, Masao,Nakamura, Mitsuaki,Morikami, Kenji,Yoshinari, Kiyoshi,Yoshida, Miyuki,Ogawa, Kotaro,Shimma, Nobuo,Tsukuda, Takuo,Ohwada, Jun
supporting information, p. 673 - 678 (2013/02/23)
Our lead compound for a phosphoinositide 3-kinase (PI3K) inhibitor (1) was metabolically unstable because of rapid glucuronidation of the phenol moiety. Based on structure-activity relationship (SAR) information and a FlexSIS docking simulation score, aminopyrimidine was identified as a bioisostere of phenol. An X-ray structure study revealed a hydrogen bonding pattern of aminopyrimidine derivatives. Finally, aminopyrimidine derivatives 33 showed strong tumor growth inhibition against a KPL-4 breast cancer xenograft model in vivo.