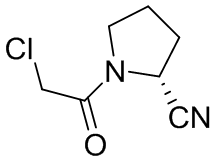
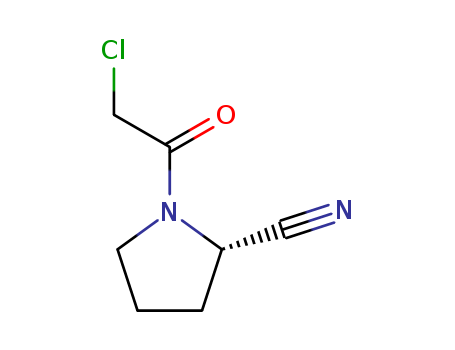
(2S)-1-(Chloroacetyl)-2-pyrrolidinecarbonitrile literature
Synthesis of main impurity of vildagliptin
Tao, Zhu,Deng, Yu,Chen, Yingjie,Wang, Anmin,Hu, Xiangnan
, p. 3489 - 3492 (2014)
A four-step synthesis of the main impurity of vildagliptin has been easily accomplished with high-yielding starting from L-proline. This compound can be used as a reference marker in an analytical method to determine the chemical purity of the vildagliptin.
Design, synthesis, biological screening, and molecular docking studies of piperazine-derived constrained inhibitors of DPP-IV for the treatment of type 2 diabetes
Kushwaha, Ram N.,Srivastava, Rohit,Mishra, Akansha,Rawat, Arun K.,Srivastava, Arvind K.,Haq, Wahajul,Katti, Seturam B.
, p. 439 - 446 (2015)
Novel piperazine-derived conformationally constrained compounds were designed, synthesized, and evaluated for in vitro Dipeptidyl peptidase-IV (DPP-IV) inhibitory activities. From a library of compounds synthesized, 1-(2-(4-(7-Chloro-4-quinolyl)piperazin-1-yl)acetyl)pyrrolidine (2g) was identified as a potential DPP-IV inhibitor exhibiting better inhibitory activity than P32/98, reference inhibitor. The in vivo studies carried out in STZ and db/db mice models indicated that the compound 2g showed moderate antihyperglycemic activity as compared to the marketed drug Sitagliptin. A two-week repeated dose study in db/db mice revealed that compound 2g significantly declined blood glucose levels with no evidence of hypoglycemia risk. Furthermore, it showed improvement in insulin resistance reversal and antidyslipidemic properties. Molecular docking studies established good binding affinity of compound 2g at the DPP-IV active site and are in favor of the observed biological data. These data collectively suggest that compound 2g is a good lead molecule for further optimization studies.
Bis-morpholinophosphorylchloride, a novel reagent for the conversion of primary amides into nitriles
Rao, P. Purnachandra,Nowshuddin, Shaik,Jha, Anjali,Rao, B. Leela Maheswara,Divi, Murali K.,Rao
supporting information, (2021/01/21)
Bis-morpholinophosphorylchloride (Bmpc), in the presence of a base, is an efficient dehydrating agent for both aromatic and aliphatic primary amides, and gives corresponding nitriles under mild conditions in god yields and purity. During the reaction the enantiomeric integrity remains intact.
Production method of vildagliptin
-
Paragraph 0079-0081; 0085-0087; 0096-0103, (2021/10/27)
and Alkali metal bromides are in vildagliptin or a salt thereof. Use of a crystal form, a deuterated substance, a tritium substitute, and a solvate as a catalyst. The vildagliptin product produced by the method is high in purity and low in impurity content, particularly the product quality control of the vildagliptin bulk drug and/or the pharmaceutical preparation is facilitated. Moreover, the reaction condition is mild, operation and control are convenient, the yield is high, the energy consumption is low, the cost is low, and the method is suitable for industrial production and popularization and application.
A facile method to synthesize vildagliptin
Zhang, Li,Jiang, Lan,Guan, Xiaoshu,Cai, Linhong,Wang, Jingyu,Xiang, Peng,Pan, Junyi,Hu, Xiangnan
, p. 305 - 309 (2020/12/01)
An efficient and high-yielding synthetic method for the preparation of vildagliptin via four steps is reported. The process starts from L-proline and involves a successful reaction with chloroacetyl chloride in tetrahydrofuran to afford (S)-1-(2-chloroacetyl)pyrrolidine-2-carboxylic acid, followed by a reaction with acetonitrile in the presence of sulfuric acid to give (S)-1-(2-chloroacetyl)pyrrolidine-2-carbonitrile. This is then reacted with 3-aminoadamantanol to give vildagliptin. 3-Aminoadamantanol is prepared from 1-aminoadamantane hydrochloride via oxidation with sulfuric acid/nitric acid and boric acid as the catalyst followed by ethanol extraction. The overall yield is 95%.
Preparation method of high chiral purity (S)-1-(2-chloracetyl) pyrrolidine-2-formonitrile
-
, (2021/08/19)
The invention discloses a preparation method of high chiral purity (S)-1-(2-chloracetyl) pyrrolidine-2-formonitrile, the preparation method comprises: S1, in the presence of a solvent and an acid-binding agent, carrying out a coupling reaction on L-proline and chloracetyl chloride to obtain an intermediate I; s2, in the presence of a solvent and sodium iodide, carrying out cyclization reaction on the intermediate I in an alkaline environment to obtain an intermediate II; s3, in the presence of a solvent and an ammonolysis agent, carrying out ammonolysis ring-opening reaction on the intermediate II to obtain an intermediate III; and S4, in the presence of a solvent and phosphorus oxychloride, carrying out chlorination and dehydration reaction on the intermediate III to obtain (S)-1-(2-chloracetyl) pyrrolidine-2-formonitrile. The method has the advantages of high chiral purity, simple steps, easily available and cheap raw materials, convenient post-treatment and high yield, and is suitable for industrial production.