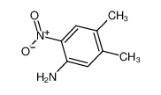
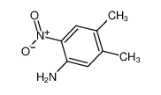
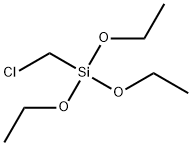
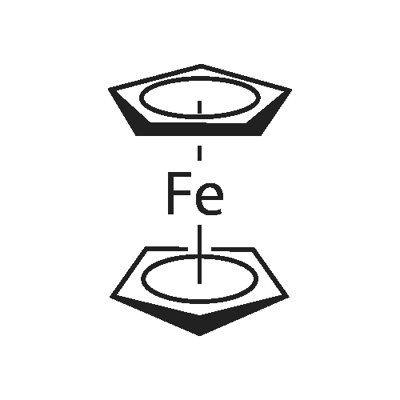
4,5-DIMETHYL-2-NITROANILINE literature
Synthesis of symmetric dinitro-functionalised troeger's base analogues
Bhuiyan, M Delower H,Mahon, Andrew B.,Jensen, Paul,Clegg, Jack K.,Try, Andrew C.
supporting information; experimental part, p. 687 - 698 (2009/07/17)
The synthesis of six new examples of 2,8-dinitro-substituted Troeger's base analogues are reported, together with the first examples of 1,7-, 3,9- and 4,10-dinitro Troeger's base analogues and the first example of a tetranitro Troeger's base compound. Several of these dinitro compounds lack substituents at the 2- and 8-positions and therefore provide further examples of Troeger's base analogues derived from anilines lacking a para substituent.
Concise total synthesis of
Buszek, Keith R.,Brown, Neil,Luo, Diheng
supporting information; experimental part, p. 201 - 204 (2009/06/20)
An efficient nine-step total synthesis of the annulated indole natural products.
Recognition properties of flavin analogues with bile acid-based receptors: Role of steric effects in hydrogen bond based molecular recognition
Chattopadhyay, Prosenjit,Nagpal, Rekha,Pandey, Pramod S.
, p. 216 - 222 (2008/09/18)
The recognition properties of 7,8-dimethyl flavin analogues by bile acid-based receptors that contain 2,6-diaminopyridine and the dioctylamide of 2,6-diaminopyridine in CHCl3 were determined. The results show that the bile acid-based receptors bind 7,8-dimethyl flavin analogues less effectively as compared to 7,8-unsubstituted flavins reported earlier, which is contrary to the known fact that the association constants increase with increasing electron-donating capacity of the substituents at the 7 and 8 positions of the flavin analogues. CSIRO 2008.
Thiourea-enhanced flavin photooxidation of benzyl alcohol
Svoboda, Jiri,Schmaderer, Harald,Koenig, Burkhard
supporting information; experimental part, p. 1854 - 1865 (2009/04/06)
Upon irradiation, flavin oxidises 4-methoxybenzyl alcohol to the corresponding aldehyde using aerial O2 as the terminal oxidant. We have observed that this reaction is significantly accelerated by the presence of thiourea. A series of thiourea-functionalised flavins has been prepared from flavin isothiocyanates and their photocatalytic efficiencies have been monitored by NMR. The alcohol photooxidation proceeds rapidly and cleanly with high turnover numbers of up to 580, exceeding previously reported performances. A likely mechanistic rationale for the more than 30-fold acceleration of the photo-redox reaction by thiourea has been derived from spectroscopic, electrochemical, and kinetic studies. Thus, thiourea acts as an electron-transfer mediator for the initial photooxidation of 4-methoxybenzyl alcohol by the excited flavins. This mechanism has similarities to electron-relay mechanisms in flavoenzymes, for which cysteine sulfenic acid intermediates are proposed. The observation that thiourea mediates flavin photo-redox processes is valuable for the design of more sophisticated photocatalysts based on Nature's best redox chromophore.