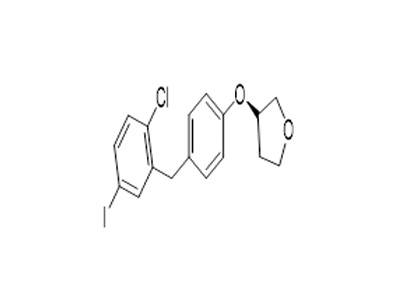
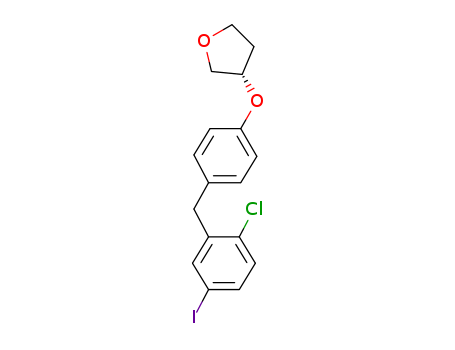
(3S)-3-[4-[(2-Chloro-5-iodophenyl)methyl]phenoxy]tetrahydro-furan literature
Preparation process of empagliflozin intermediate
-
, (2021/07/17)
The invention discloses a preparation process of an empagliflozin intermediate, and relates to the technical field of biological pharmacy, the process comprises the following steps: taking a compound of a formula d structure and s-3-hydroxytetrahydrofuran (namely a compound e) as initial raw materials, dehydrating to obtain a compound of a formula c structure; sequentially adding alkali metal and a chlorination reagent to obtain a compound b; further performing Friedel-Crafts reaction with a compound with a structure as shown in a formula f under the catalytic action of Lewis acid to synthesize a compound with a structure as shown in a formula a; and finally, carrying out reduction reaction to obtain a target compound with a structure shown in a formula g. The preparation method is low in cost, easy to operate, less in wastewater, easy in post-treatment, high in yield, high in product purity and suitable for industrial production.
Empagliflozin intermediate preparation method
-
, (2020/02/08)
The invention discloses an empagliflozin intermediate preparation method, which comprises: carrying out substitution by using p-methoxybenzyl chloride and p-iodoaniline as starting raw materials to obtain a compound IV, performing diazotization and a Sandmeyer reaction to obtain a compound III, further performing demethylation under the action of boron tribromide to obtain a compound II, and finally performing condensation with (S)-3-p-toluenesulfonyloxy tetrahydrofuran to obtain the target compound I, wherein the product purity is greater than or equal to 99.0%. According to the invention, the preparation method has advantages of simple and easily available raw materials, low cost, simple operation steps, simple post-treatment and high product yield, and is suitable for industrial production.
Design, synthesis and anticancer activities of halogenated Phenstatin analogs as microtubule destabilizing agent
Hu, Shengquan,Sun, Wuji,Wang, Yeming,Yan, Hong
, p. 465 - 472 (2019/02/09)
A series of halogenated Phenstatin analogs were designed as microtubule destabilizing agent by docking study. It was synthesized within three steps starting from 2-chloro-5-iodobenzoic acid and substituted benzene. All the products were characterized by 1H NMR and 13C NMR spectral analysis, and the stereochemical structure was also confirmed by a single crystal X-ray diffraction crystallographic analysis. The microtubule destabilizing activities were evaluated in vitro with human liver cancer Huh-7 cell line and human lung cancer A549 cell line. Some of the HPAs were achieved IC50 about 5.0 μM against human liver cancer Huh-7 cells. [Figure not available: see fulltext.].
Synthesis method of Empagliflozin intermediate
-
Paragraph 0009; 0033; 0036, (2018/07/07)
The invention discloses a synthesis method of an Empagliflozin intermediate. The synthesis method uses 4-hydroxybenzyl chloride as a starting material to sequentially react with methanesulfonyl chloride and (S)-3-hydroxytetrahydrofuran to obtain compound III, then react with 4-iodoaniline to obtain compound IV, and finally react with cuprous chloride after diazotization to obtain (S)-3-(4-(5-iodine-2-chlorobenzyl)phenoxy) tetrahydrofuran. The raw materials used in the synthesis method are simple and easy to obtain, the operation steps are simple, the post-treatment is simple, the product yieldis high, and the method is suitable for industrial production.