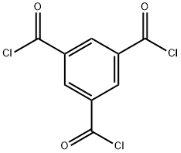
1,3,5-Benzenetricarboxylic acid chloride literature
Synthesis and geometric structure of N, N, N'-tris(Benzoyl)trimesinic acid dihydrazide
Cai, Yan-Hua,Zhao, Li-Sha,Ren, Li-Ping,Wang, Feng
, p. 9356 - 9358 (2013)
N, N, N'-Tris(benzoyl) trimesinic acid dihydrazide was synthesized from benzoyl hydrazine and trischloride and the structures of N,N,N'- tris(benzoyl) trimesinic acid dihydrazide had been characterized by Fourier transform infrared spectroscopy, 1H nuclear magnetic resonance techniques. The reaction condition was investigated by orthogonal experiment and the optimized reaction condition by orthogonal experiment is molar ratio of benzoyl hydrazine: trischloride 5:1, reaction time 4 h, reaction temperature 70°C, the yield is 65.7 %. SEM showed the particles of N,N,N'-tris(benzoyl)trimesinic acid dihydrazide were not completely regular and the average size of the particles was 3-4 μm. At the same time, the optimized geometric structure of N,N,N'-tris(benzoyl)trimesinic acid dihydrazide was carried out by theoretical calculations using the semiempirical method PM3.
Star-Shaped Conjugated Molecules with Oxa- or Thiadiazole Bithiophene Side Arms
Kotwica, Kamil,Kostyuchenko, Anastasia S.,Data, Przemyslaw,Marszalek, Tomasz,Skorka, Lukasz,Jaroch, Tomasz,Kacka, Sylwia,Zagorska, Malgorzata,Nowakowski, Robert,Monkman, Andrew P.,Fisyuk, Alexander S.,Pisula, Wojciech,Pron, Adam
, p. 11795 - 11806 (2016)
Star-shaped conjugated molecules, consisting of a benzene central unit symmetrically trisubstituted with either oxa- or thiadiazole bithiophene groups, were synthesized as promising molecules and building blocks for application in (opto)electronics and electrochromic devices. Their optical (Eg(opt)) as well as electrochemical (Eg(electro)) band gaps depended on the type of the side arm and the number of solubilizing alkyl substituents. Oxadiazole derivatives showed Eg(opt) slightly below 3 eV and by 0.2 eV larger than those determined for thiadiazole-based compounds. The presence of alkyl substituents in the arms additionally lowered the band gap. The obtained compounds were efficient electroluminophores in guest/host-type light-emitting diodes. They also showed a strong tendency to self-organize in monolayers deposited on graphite, as evidenced by scanning tunneling microscopy. The structural studies by X-ray scattering revealed the formation of supramolecular columnar stacks in which the molecules were organized. Differences in macroscopic alignment in the specimen indicated variations in the self-assembly mechanism between the molecules. The compounds as trifunctional monomers were electrochemically polymerized to yield the corresponding polymer network. As shown by UV/Vis-NIR spectroelectrochemical studies, these networks exhibited reversible electrochromic behavior both in the oxidation and in the reduction modes.
Uncommon hexagonal microtubule based gel from a simple trimesic amide
Shi, Naien,Yin, Gui,Li, Hongbian,Han, Min,Xu, Zheng
, p. 2011 - 2015 (2008)
A small molecule of N,N′,N″-tris(3-methylpyridyl)-trimesic amide was assembled into a novel hexagonal microtube through intermolecular hydrogen bonds, and simultaneously formed a gel system in H2O-THF mixed solvent. Tuning gelator concentration or the preparation method can effectively control the size of the hexagonal tubes. The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2008.
Binding of Heptanedioic Acid to a Threefold Pyridine Arylamide Receptor. Enhancement of the Stability of Supramolecular Solution Structures by Multiple Binding Sites
Koenig, Burkhard,Moeller, Oliver,Bubenitschek, Peter,Jones, Peter G.
, p. 4291 - 4293 (1995)
-
Cobalt Metallogel Interface for Selectively Sensing l -Tryptophan among Essential Amino Acids
Malviya, Novina,Sonkar, Chanchal,Ganguly, Rakesh,Mukhopadhyay, Suman
, p. 7324 - 7334 (2019)
The development of metallogels widens the span of sensing activity as it opens new opportunities to develop chemosensors through metal-ligand interactions. Herein, a new nitrile-substituted 1,3,5-tricarboxamide-based gelator G4 has been fabricated and shows aggregate-induced enhanced emission (AIEE) after gelation in the presence of water. A dimethylformamide (DMF) solution of the gelator shows rapid crystallization, but addition of water to a DMF solution of gelator G4 leads to gelation at room temperature. In addition, gelator G4 was used for the formation of metallogels, and among them, the cobalt metallogel has been found to be effective for sensing l-tryptophan in the gel state through the quenching of AIEE. Interestingly, the gel is also effective in sensing bovine serum albumin protein at the nanomolar level, which contains an l-tryptophan residue. The limit of detection of Co(II)G4 for selective sensing of tryptophan has been found to be 2.4 × 10-8 M. To the best of our knowledge, there have been no reports to date of a metallogel being utilized to discriminate and selectively sense an amino acid and a protein. The gelation properties of the organic gelator molecule and metallogels have been explored through various spectroscopic tools and physicochemical experiments.
Tuning of Bistability, Thermal Stability of the Metastable States, and Application Prospects in the C3-Symmetric Designs of Multiple Azo(hetero)arenes Systems
Gupta, Debapriya,Gaur, Ankit Kumar,Kumar, Pravesh,Kumar, Himanshu,Mahadevan, Anjali,Devi, Sudha,Roy, Saonli,Venkataramani, Sugumar
supporting information, p. 3463 - 3472 (2021/01/21)
Light-responsive molecular systems with multiple photoswitches in C3-symmetric designs have enormous application potential. The design part of such molecular systems is critical due to its influence in several properties associated with the photoswitches. In order to tune, and in the evaluation of the design–property relationship, we synthesized 18 tripodal systems with variations in the core, linkers, connectivity, and azo(hetero)arene photoswitches. Through extensive spectroscopic and computational studies, we envisaged the factors controlling near-quantitative photoisomerization in both the directions (bistability) and the thermal stability of the metastable states. Furthermore, we also evaluated the impact of designs in obtaining reversible photo-responsive sol-gel phase transitions, solvatochromism, photo- and thermochromism.
Br?nsted acid-catalyzed chlorination of aromatic carboxylic acids
Yu, Zhiqun,Yao, Hongmiao,Xu, Qilin,Liu, Jiming,Le, Xingmao,Ren, Minna
supporting information, p. 685 - 689 (2021/04/09)
The chlorination of aromatic carboxylic acids with SOCl2 has been effectively performed by reacting with a Br?nsted acid as the catalyst. Based on this discovery, an efficient catalytic method that is cheaper than traditional catalytic methods was developed. 20 substrates were chlorinated offering excellent yields in a short reaction time. And the SOCl2/Br?nsted acid system has been used in a larger scale preparative reaction. A dual activation mechanism was proposed to prove the irreplaceable system of SOCl2/Br?nsted acid.
Instant Photochromism Caused by Radical Formation in Photocatalytic Decarboxylation of Dihydrothiazole Derivative?
Xu, Zhen,Malik, Abaid Ullah,Shu, Mouhai,Cui, Yong
, p. 2774 - 2780 (2021/08/09)
A pair of new enantiomeric compounds, (R)/(S)-1,3,5-benzene-triyl-2,2',2”-tris(4,5-dihydrothiazole-4-carboxylic acid) (H3LRRR and H3LSSS) are synthesized in one step synthetic route with high yield. Instant photochromism has been investigated to elaborate the photocatalytic decarboxylation of the dihydrothiazole derivative by electron paramagnetic resonance spectroscopy (EPR), photoluminescence (PL), FT-IR, high resolution mass spectra, X-ray photoelectron spectroscopy and UV-Vis spectroscopic techniques. The results indicate that the photochromic transformation is originated from the formation of the radical during the photocatalytic decarboxylation of the 4,5-dihydrothiazole-4-carboxylic acid units.
Citrate plasticizer
-
Paragraph 0061; 0064; 0066; 0069, (2021/05/15)
The invention discloses a citrate plasticizer and a preparation method thereof, and the preparation method of citrate comprises the following steps: firstly, citric acid and alcohol react to prepare citric acid triester, and then dianhydride acylation is carried out to prepare carboxyl-containing citric acid triester. After chlorination, the carboxyl-containing citric acid triester and the tris(2-ethoxyl) isocyanurate are subjected to esterification reaction to prepare triester citrate containing the isocyanurate. According to the present invention, the preparation method has characteristics of simple operation, wide raw material source and mild reaction condition, and meets the industrial production, the prepared triester citrate containing isocyanurate has a good plasticizing effect and excellent thermal stability, low-temperature flexibility, solvent extraction resistance, migration resistance and flame retardance, and can be widely applied to plastic rubber plasticizers.