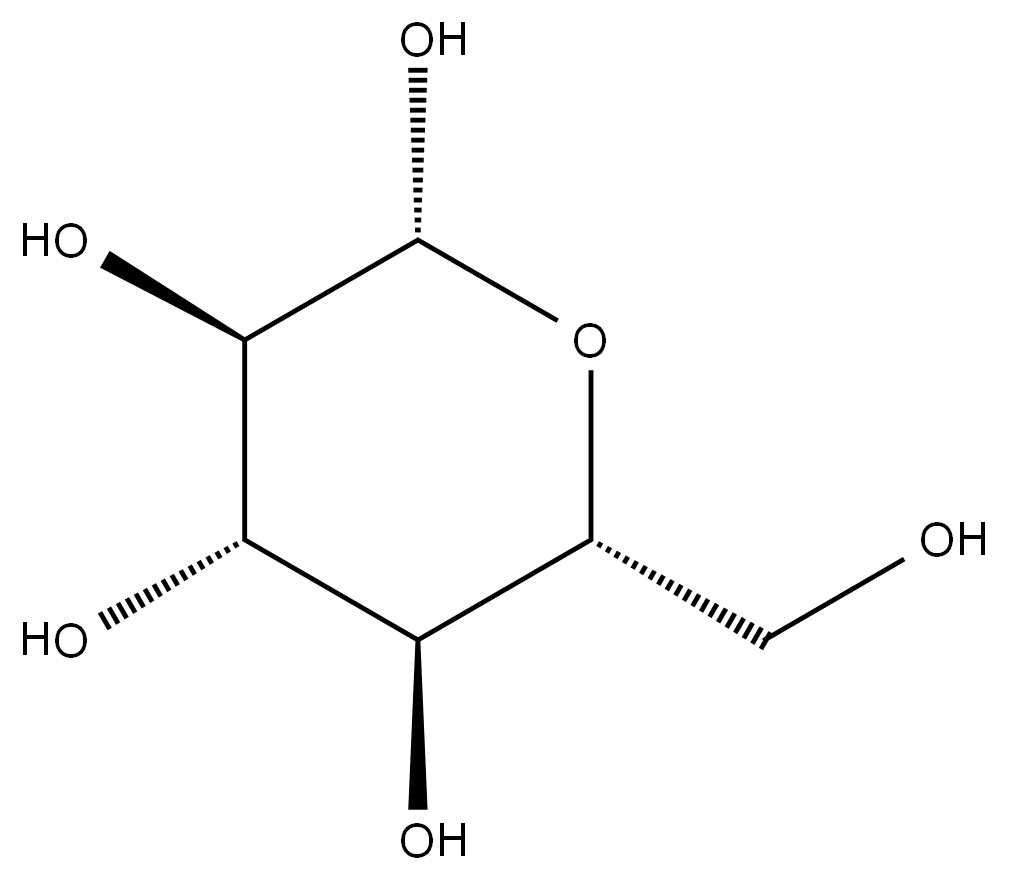
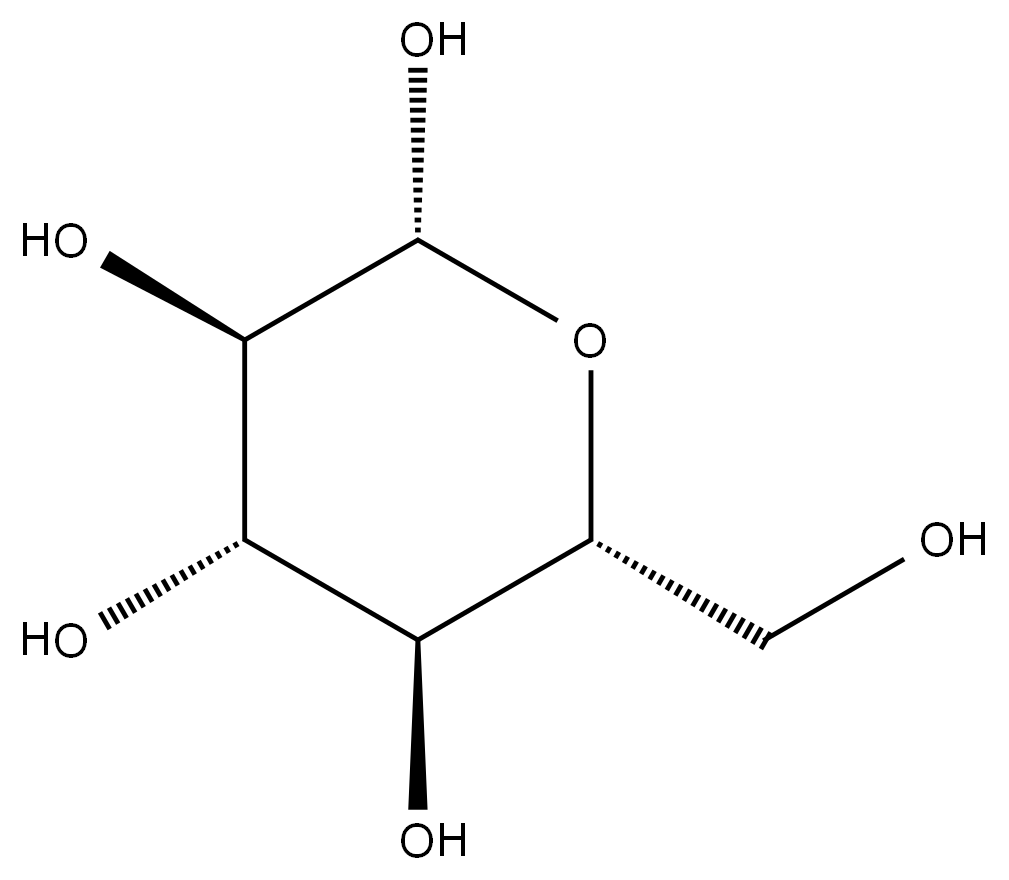
DEXTROSE literature
Three novel quassinoids, javanicolides A and B, and javanicoside A, from seeds of Brucea javanica
Kim, Ik Hwi,Suzuki, Ryoko,Hitotsuyanagi, Yukio,Takeya, Koichi
, p. 9985 - 9989 (2003)
Two novel quassinoids, javanicolides A and B, and one novel quassinoid glucoside, javanicoside A were isolated from the seeds of Brucea javanica Merr. (Simaroubaceae), along with four known quassinoids, yadanziolides A and D, and bruceins D and E, and two known quassinoid glucosides, yadanziosides D and L. Their structures were elucidated by the analysis of spectral data and chemical evidence.
A new megastigmane glucoside and three new flavonoid glycosides from spiraea prunifolia var. simpliciflora
Yean, Min Hye,Kim, Ju Sun,Kang, Sam Sik,Kim, Yeong Shik
, p. 1123 - 1131 (2014)
A new megastigmane glucoside, simplicifloranoside (1), and three new flavonol glycosides, prunifolianosides A-C (2-4, resp.), were isolated from the aerial parts of Spiraea prunifolia var. simpliciflora. In addition, fifteen known compounds, including five phenolic acids, three lignans, four flavonoids, one eugenol glycoside, and two alkyl-primeverosides, were also identified. Their structures were elucidated on the basis of detailed spectroscopic analyses and acid hydrolysis. Copyright
Two new bufadienolides and one new pregnane from Helleborus thibetanus
Zhang, Hui,Su, Yanfang,Yang, Fengying,Zhao, Zeqing,Gao, Xiumei
, p. 164 - 167 (2014)
Two new bufadienolides, 3β,14β,16β-trihydroxy-5α-bufa-20,22-dienolide (1) and 14β-hydroxy-3β-[β-D-glucopyranosyl-(1→6)-(β-D-glucopyranosyl)oxy]-5α-bufa-20,22-dienolide (2), one new pregnane, 3β-hydroxypregna-5,16-diene-20-one-1β-yl sulfate (3), along with
Flavonoids from Malus hupehensis and their cardioprotective effects against doxorubicin-induced toxicity in H9c2 cells
Wang, Shu-Qi,Zhu, Xiao-Feng,Wang, Xiao-Ning,Shen, Tao,Xiang, Feng,Lou, Hong-Xiang
, p. 119 - 125 (2013)
Three biflavonoid glycosides along with 12 known flavonoids, were isolated from leaves of Malus hupehensis. The complete structures of two of the compounds were established from analysis of MS, NMR spectroscopic and CD data, as well as DFT CD calculations they were determined to be atropisomeric along a central biaryl axis. The antioxidant activities and protective effects of the compounds against doxorubicin-induced cardiomyopathy in H9c2 cells were also investigated. Amongst all of the isolated compounds, quercetin was the most active radical scavenger with EC50 values of 3.2 μM and 17.8 μM by the DPPH and ABTS+ methods, respectively. The results indicated that three of the flavanoids also had a strong protective influence against doxorubicin-induced cell death with EC50 values of 8.3, 5.2 and 7.6 μM, respectively.
Alkenes with antioxidative activities from Murraya koenigii (L.) Spreng
Ma, Qin-Ge,Xu, Kun,Sang, Zhi-Pei,Wei, Rong-Rui,Liu, Wen-Min,Su, Ya-Lun,Yang, Jian-Bo,Wang, Ai-Guo,Ji, Teng-Fei,Li, Lu-Jun
, p. 799 - 803 (2016)
Four new alkenes (1-4), and six known alkenes (5-12) were isolated from Murraya koenigii (L.) Spreng. Their structures were elucidated on the basis of spectroscopic analyses and references. Compounds (1-12) were evaluated for antioxidative activities. Amo
Eremophilane derivatives from Senecio dianthus
Huang, Shuai,Zhou, Xian-Li,Wang, Hong-Yan,Wang, Cui-Juan,Zhou, Xiao-Li,Xiao, Feng,Weng, Jie
, p. 711 - 718 (2013)
From the aerial parts of Senecio dianthus, five eremophilane glucosides (1, 2, 4-6) and one new eremophilenolide (7) were isolated, together with sixteen known compounds (3, 8-22). Their structures and relative configurations were elucidated on the basis of extensive spectroscopic analysis, including HR-ESI-MS, X-ray, CD, 1D- and 2D-NMR experiments.
Monoterpene derivatives with anti-allergic activity from red peony root, the root of Paeonia lactiflora
Shi, Yan-Hong,Zhu, Shu,Ge, Yue-Wei,He, Yu-Min,Kazuma, Kohei,Wang, Zhengtao,Yoshimatsu, Kayo,Komatsu, Katsuko
, p. 55 - 61 (2016)
The methanolic extract and its subfractions from red peony root, the dried roots of Paeonia lactiflora Pallas showed potent antiallergic effects, as inhibition of immunoglobulin E (IgE)-mediated degranulation in rat basophil leukemia (RBL)-2H3 cells. Bioassay-guided fractionation led to the isolation of 16 monoterpene derivatives, including 3 new compounds, paeoniflorol (1), 4′-hydroxypaeoniflorigenone (2) and 4-epi-albiflorin (3), together with 13 known ones (4-16). The chemical structures of the new compounds were elucidated on the basis of spectroscopic and chemical evidences. Among the isolated monoterpene derivatives, nine compounds showed potent anti-allergic effects and compound 1 was the most effective. A primary structure-activity relationship of monoterpene derivatives was discussed.
Cytisine-type alkaloids and flavonoids from the rhizomes of Sophora tonkinensis
Pan, Qi-Ming,Zhang, Gui-Jie,Huang, Ri-Zhen,Pan, Ying-Ming,Wang, Heng-Shan,Liang, Dong
, p. 429 - 435 (2016)
Abstract: A new cytisine-type alkaloid, (?)-N-hexanoylcytisine (1), and a new isoflavan, (3S, 4R)-4-hydroxy-7,4′-dimethoxyisoflavan 3′-O-β-d-glucopyranoside (2), along with 10 known compounds, were isolated from the rhizomes of Sophora tonkinensis. Their structures were determined by spectroscopic methods, chemical evidence, and ECD data analysis. All of the isolates were evaluated for their cytotoxic activities against four human tumor cell lines.
Two new glycosidal metabolites of endophytic fungus Penicillium sp. (NO.4) from Tapiscia sinensis
Wan, Qiao,Feng, Ziwei,Li, Xueshuang,Lv, Mengmeng,Guo, Zhiyong,Deng, Zhangshuang,Zou, Kun
, p. 283 - 286 (2016)
Two new glycosides, 8-O-β-D-glucopyranosyl-6-methyl-1-carboxylate methyl ester xanthone (1) and 4'-O-β-d-galactopyranosyl djalonensone (2), together with four known compounds, 8-hydroxy-6-methyl-9-oxo-9H-xanthene-1-carboxylate methyl ester (3), cassionllin (4), djalonensone (5) and alternariol (6), were isolated from the endophytic fungus Penicillium sp. (NO.4) of Tapiscia sinensis Oliv. The structures of compounds 1-6 were elucidated by the analysis of 1D and 2D NMR and HRMS. The cytotoxic activities of these compounds were evaluated against four cancer cell lines, as well as antimicrobial activities against two plant-pathogenic microbes. Compounds 1-6 showed moderate cytotoxicity against the A549 cancer cell line with IC50values ranging from 6.8 to 35.8 μg mL-1and were found to be inactive against three other cancer cell lines MCF-7, Caski and Hep G-2.
Furofuran Lignan Glucosides with Estrogen-Inhibitory Properties from the Bangladeshi Medicinal Plant Terminalia citrina
Muhit, Md. Abdul,Umehara, Kaoru,Mori-Yasumoto, Kanami,Noguchi, Hiroshi
, p. 1298 - 1307 (2016)
Extracts from the leaves of the Bangladeshi medicinal plant Terminalia citrina were prepared, and 13 new furofuran lignan glucosides, terminalosides A-K (1-4, 6-12), 2-epiterminaloside D (5), and 6-epiterminaloside K (13), were characterized using various spectroscopic techniques. Twelve of the isolates were found to contain rare tetraoxygenated aryl groups in their structures. Analysis of the NMR chemical shifts for the oxymethine signals in the furofuran ring suggested a pragmatic approach to determining the relative configuration of these compounds. The ECD and NOESY spectroscopic data obtained allowed for the deduction of the absolute configurations and conformations of the compounds. The isolates were tested for their estrogenic/antiestrogenic activity using the MCF-7 and T47D estrogen-responsive human breast cancer cell lines. Terminalosides B (2) and G (8) exhibited inhibitory effects for both cell lines, and estradiol-enhanced cell proliferation was suppressed by 90% at concentrations lower than 10 M. Terminaloside E (6) showed inhibitory activity against the T47D cell line, whereas terminalosides C (3), F (7), and I (10) and 6-epiterminaloside K (13) displayed antiestrogenic activity against MCF-7 cells.
Flavone tetraglycosides and benzyl alcohol glycosides from the Mongolian Medicinal plant dracocephalum ruyschiana
Selenge, Erdenechimeg,Murata, Toshihiro,Kobayashi, Kyoko,Batkhuu, Javzan,Fumihiko Yoshizaki
, p. 186 - 193 (2013)
From an extract of the aerial parts of Dracocephalum ruyschiana, five new flavone tetraglycosides, five new benzyl alcohol glycosides, and 19 known compounds were isolated. The tetraglycosides contain a 7-O-β-D- glucopyranosyl-(1→2)-β-D-glucopyranosyl-( 1→2)-[α-L- rhamnopyranosyl-(1→6)]-β-D-glucopyranosyl moiety. The benzyl alcohol glycosides had acyl groups on their glycosyl or aglycone moieties. The compounds were tested for antioxidant activity using DPPH. Although the new compounds were not active, phenylpropanoylquinic acid derivatives were revealed as radical scavengers in D. ruyschiana.
An effective cellulose-to-glucose-to-fructose conversion sequence by using enzyme immobilized Fe3O4-loaded mesoporous silica nanoparticles as recyclable biocatalysts
Lee, Yi-Chun,Chen, Ching-Tien,Chiu, Yu-Ting,Wu, Kevin C.-W.
, p. 2153 - 2157 (2013)
-
A C14-polyacetylenic glucoside with an α-pyrone moiety and four C10-polyacetylenic glucosides from Mediasia macrophylla
Kurimoto, Shin-ichiro,Okasaka, Mamoru,Kashiwada, Yoshiki,Kodzhimatov, Olimjon K.,Takaishi, Yoshihisa
, p. 688 - 692 (2010)
Polyacetylenic glucosides (1-5) were isolated from the MeOH extract of Mediasia macrophylla, and their structures were established by spectroscopic analyses. Compounds 2-4 were the first examples of C10-polyacetylenic glucosides found in the family Umbelliferae, while compound 1 was a unique polyacetylenic glucoside possessing an α-pyrone moiety.
Chemical constituents of the roots of Codonopsis lanceolata
Du, Young Eun,Lee, Jin Su,Kim, Hye Mi,Ahn, Ji-Hye,Jung, In Ho,Ryu, Jong Hoon,Choi, Jung-Hye,Jang, Dae Sik
, p. 1082 - 1091 (2018)
A new phenylpropanoid (1), a new alkaloid (11), and a new natural polyacetylene (17), together with nine phenolic compounds (2–10), five alkaloids (12–16), three polyacetylenes (18–20), three triterpenoidal saponins (21–23), one phenylethanoid glycoside (24), and three hexyl glycosides (25–27) with previous known structures, were isolated from the roots of Codonopsis lanceolata. All of the isolates 1–27 were evaluated for their inhibitory effects on LPS-induced nitric oxide (NO) production in RAW 264.7 macrophages and cell viability in A2780 human ovarian cancer cells. Among the isolates, lancemasides A and B have a significant inhibitory effect on the production of NO in RAW264.7 cells (IC50 values < 50?μM). In A2780 cells, lancemaside A exhibited the most potent inhibitory effect on cell viability. This is the first report on the pharmacological activities of lancemaside B (22).
Rapulasides A and B: Two novel intermolecular rearranged biiridoid glucosides from the roots of Heracleum rapula
Xiao, Weilie,Li, Shenghong,Niu, Xuemei,Zhao, Yu,Sun, Handong
, p. 5743 - 5746 (2005)
Two novel intermolecular rearranged biiridoid glucosides, rapulasides A and B (1 and 2), have been isolated from the roots of Heracleum rapula. Their structures were identified by extensive spectral analysis especially different NMR techniques. NOESY experiment, with the help of Dreiding molecular model, was used to elucidate their relative stereochemistry. Both compounds were tested for their inhibitory effects on rabbit platelet aggregation induced by PAF, ADP, or AA, respectively. Only trends of inhibition were observed for them.
Phytochemical Studies on Two Australian Anigozanthos Plant Species
Hendra, Rudi,Keller, Paul A.
, p. 2141 - 2145 (2017)
Phytochemical studies of two Australian Anigozanthos (kangaroo paw) species, A. rufus and A. pulcherrimus, resulted in the identification of 13 secondary metabolites. 2-Amino-6-O-p-coumarylheptanedioic acid (3) and chalcone-5′-O-(4-O-p-coumaryl)-O-β-d-glucopyranoside (12) are reported as new compounds and are accompanied by nine flavonoids (2, 5-11, 13) and two anthocyanins (1, 4). Compounds 1 and 4 were isolated as red solids from A. rufus and are likely responsible for the coloration of the flowers. Compounds 1, 3, and 6 showed weak antimicrobial activities against Acinetobacter baumannii ATCC 19606 at concentrations of 52.4, 94.9, and 53.9 μM, respectively.
Cytotoxic phenylpropanoid glycosides from Cirsium japonicum
Shang, Dong-Li,Ma, Qin-Ge,Wei, Rong-Rui
, p. 1122 - 1130 (2016)
Three new phenylpropanoid glycosides 1–3, along with nine known phenylpropanoid glycosides 4–12, were isolated from the aerial parts of Cirsium japonicum. The structures of isolated compounds were elucidated by chemical and spectroscopic methods. Compounds 1, 3, 6, 8, and 11 showed moderate cytotoxicities against MCF-7, U87, HCT116, and A549 cell lines with IC50values in the range of 1.35–11.32?μM. The known compounds 4–12 were obtained from this plant for the first time.
Phytochemical investigation of the fruit peels of Citrus reticulata Blanco
Khan, M. Aasim,Ali,Alam, Prawez
, p. 610 - 620 (2010)
Phytochemical investigation of the fruit peels of Citrus reticulata Blanco (Rutaceae) resulted in the isolation of three new phytoconstituents along with n-hexacosonoic acid. Their structures have been established as 18H-urs-5,11-dien-3-ol-11-one-3-D-gluc
Isolation, identification and bioactivities of abietane diterpenoids from: Premna szemaoensis
Pu, De-Bing,Wang, Ting,Zhang, Xing-Jie,Gao, Jun-Bo,Zhang, Rui-Han,Li, Xiao-Nian,Wang, Yong-Mei,Li, Xiao-Li,Wang, He-Yao,Xiao, Wei-Lie
, p. 6425 - 6435 (2018)
Investigation of the leaves and stems of Premna szemaoensis resulted in the isolation of twelve new abietane diterpenoids, szemaoenoids A-L (1-12), together with four known abietane diterpenoids (13-16). The structures involved two rearranged-abietane skeletons: 17(15 → 16)-abeo-abietane (7, 10-12, 14 and 15) and 17(15 → 16),18(4 → 3)-diabeo-abietane (1-6, 13 and 16). The structures of the new compounds were established mainly by analyzing NMR and HRESIMS data. The absolute configurations of 1, 3 and 10 were confirmed by single crystal X-ray diffraction analysis. In bioactivity assays, compounds 11, 12, 14 and 15 were active against two human colon cancer cell lines (HCT-116 and HT-29) with IC50 values ranging from 8.8 to 34.3 μM, and compounds 10, 13 and 14 exhibited effective free radical scavenging activity with IC50 values ranging from 35.6 to 41.5 μM by DPPH experiment.
Antihepatotoxic, nephroprotective, and antioxidant activities of phenolic compounds from Satureja macrostema leaves against carbon tetrachloride-induced hepatic damage in mice
Gutierrez, Rosa Martha Perez
, p. 1846 - 1855 (2013)
Satureja macrostema (SM) is used with culinary and medicinal purposes. Methanol extract from SM was investigated for its phenolic content, antioxidant, hepatoprotective, and kidney protective activities. Liver and kidney damage were induced in rats with CCl4. Hepatoprotective efficacy was measured by the activity of aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, total bilirubin, cholesterol, high density lipoprotein and total protein, and lipid peroxidation. Kidney function was evaluated by measuring plasma urea and creatinine. Antioxidant activity was evaluated by measuring blood glutathione content, superoxide dismutase and catalase activities, and malondialdehyde equivalent; their activity was comparable to that of silymarin, a well-known hepatoprotective agent. Methanol extracts of S. macrostema showed potent antioxidant, kidney protective, and hepatoprotective activities; in-depth chromatographic investigation resulted in the identification of six new flavonoid glycosides: 5-hydroxy-3,6,4′- trimethoxyflavonol-7-C-α-l-rhamnopyranosyl-(1 → 3)-β-d- glucopyranoside (2), 4′-methoxy-5,7,3′,5′-tetra- hydroxyflavanone-3-O-β-d-rhamnopyranosyl-(1 → 2)-β-d- rhamnopyranoside (3), 5,4′-dimethoxy-7,3′,5′- trihydroxyflavanone-3-O-β-d-rhamnopyranoside (4), 5,3′,4′, 5′-tetrahydroxyflavanone-7-O-β-d-rhamnopyranoside (5), 5,3′,4′,5′-tetramethoxyflavanone-7-O-β-d- rhamnopyranoside (6), and 5,4′-dimethoxy-3′-hydroxyflavone-7-β- d-rhamnopyranoside (8) along with three known compounds: 5-hydroxy-7,4′- dimethoxyflavone (1), prunin (7), and diosmin (9) that were isolated. Structural elucidation of the new compounds was established based on the spectral data. The present study revealed that S. macrostema leaves have a significant radical scavenging and hepatoprotective activity.
A novel hexanordammarane glycoside from the leaves and stems of Panax quinquefolium L.
Liu, Jin-Ping,Lu, Dan,Li, Ping-Ya
, p. 744 - 748 (2012)
One novel hexanordammarane glycoside, ginsenoside R10, was isolated from the leaves and stems of Panax quinquefolium L. as a minor constituent. It is the first time that a hexanordammarane glycoside isolated from the plant of Panax quinquefolium L. Its structure was elucidated as 3-O-β-D-glucopyranosyl-3β, 12β-dihydroxy-22, 23, 24, 25, 26, 27-hexanordammarane-20-one (1), by the combination analysis of one-dimensional NMR, two-dimensional NMR and mass spectrometry.
Hydrolysis of cellulose in 1-allyl-3-methylimidazolium chloride catalyzed by methyltrioxorhenium
Yuan, Yuguo,Wang, Jingyun,Fu, Nihong,Zang, Shuliang
, p. 46 - 49 (2016)
Methyltrioxorhenium (MTO) has been applied as catalyst to promote cellulose hydrolysis by using the ionic liquid 1-allyl-3-methylimidazolium chloride ([Amim]Cl) as solvent. When using 7 mol% of MTO, 70 μL of water, ca. 0.6 mmol of microcrystalline cellulose and 2.0 g of [Amim]Cl under microwave irradiation for 30 min at 150 °C, 51.2% of total reducing sugar (TRS) and 24.7% of glucose yield can be obtained. The nucleophilic attack of electron-rich O atom of β-1,4-glycosidic bond to electron-poor Re atom of MTO, leading to the broken of β-1,4-glycosidic bond, is assumed to be crucial for cellulose degradation.
Flavonoids from silkworm droppings and their promotional activities on heme oxygenase-1
Park, Ji-Hae,Lee, Dae-Young,Yun, Pyeong,Yeon, Seung-Woo,Ko, Jong Hee,Kim, Yong-Soon,Baek, Nam-In
, p. 377 - 382 (2011)
A new flavane glucoside, 7,2'-dihydroxy-8-hydroxyethyl-4'-methoxyflavane- 2'-O-β-d-glucopyranoside (3), along with three known flavonoids, 7,2'-dihydroxy-8-prenyl-4'-methoxyflavane (1), euchrenone a7 (2), and 7,2'-dihydroxy-8-prenyl-4'-methoxy-2'-O-β-d-glucopyranosylflavane (4), was isolated from silkworm droppings. The structures of the compounds were elucidated on the basis of 1D and 2D NMR spectroscopic analyses and optical rotational characteristics. The compounds isolated from silkworm droppings were evaluated for their effects on heme oxygenase-1 (HO-1) activity. Compounds 1 and 3 increased the expression of HO-1 in HepG2 cells. HO-1 is an antioxidant enzyme that catabolizes heme to carbon monoxide, free iron, and biliverdin, all of which are involved in the suppression of inflammatory mediators.
Four new monoterpenoid glycosides from the flower buds of Magnolia biondii
Feng, Wei-Sheng,He, Yu-Huan,Zheng, Xiao-Ke,Wang, Jian-Chao,Cao, Yan-Gang,Zhang, Yan-Li,Song, Kai
, (2016)
Four new monoterpenoid glycosides 1-4, named magnoliaterpenoid A-D, were isolated from a 50% aqueous acetone extract of flower buds of Magnolia biondii, along with one known compound, (1′R,3′S,5′R,8′S,2Z,4E)-dihydrophaseic acid 3-O-β-D-glucopyranoside (5). Their structures and relative configuration were identified by extensive spectroscopic analysis (IR, UV, MS, 1D and 2D NMR). The aglycones of these four new compounds possess seven-membered rings systems, which are very rare. A plausible biosynthetic route for the four new compounds was proposed via the biogenetic isoprene rule. Compounds 1, 2, 3, and 4 showed no antimicrobial activity at the concentration range of 1.95-250 μg/mL.
Osteosaponins 1 and 2: Two new saponin glycosides from Osteospermum vaillantii
Ahmed, Bahar,Khan, Riaz A.,Al-Howiriny, Tawfeq A.,Al-Rehaily, Adnan J.
, p. 1258 - 1267 (2010)
Osteospermum vaillantii (Decne) T. Norl., collected from southern Saudi Arabia, yielded two new saponins characterised as 3-O-β-D-glucopyranosyl- (2′ → 1″)-β D-glucopyranosyl-(3′ → 1?)-O-β D-galactopyranosyl-oleanolic acid, designated as osteosaponin (1),
Regeneration of cello-oligomers via selective depolymerization of cellulose fibers derived from printed paper wastes
Voon, Lee Ken,Pang, Suh Cem,Chin, Suk Fun
, p. 31 - 37 (2016)
Cellulose extracted from printed paper wastes were selectively depolymerized under controlled conditions into cello-oligomers of controllable chain lengths via dissolution in an ionic liquid, 1-allyl-3-methylimidazolium chloride (AMIMCl), and in the presence of an acid catalyst, Amberlyst 15DRY. The depolymerization process was optimized against reaction temperature, concentration of acid catalyst, and reaction time. Despite rapid initial depolymerization process, the rate of cellulose depolymerization slowed down gradually upon prolonged reaction time, with 75.0 wt% yield of regenerated cello-oligomers (mean Viscosimetric Degree of Polymerization value of 81) obtained after 40 min. The depolymerization of cellulose fibers at 80 °C appeared to proceed via a second-order kinetic reaction with respect to the catalyst concentration of 0.23 mmol H3O+. As such, the cellulose depolymerization process could afford some degree of control on the degree of polymerization or chain lengths of cello-oligomers formed.
Isolation and evaluation of kaempferol glycosides from the fern Neocheiropteris palmatopedata
Yang, Jian-Hong,Kondratyuk, Tamara P.,Marler, Laura E.,Qiu, Xi,Choi, Yongsoo,Cao, Hongmei,Yu, Rui,Sturdy, Megan,Pegan, Scott,Liu, Ying,Wang, Li-Qin,Mesecar, Andrew D.,Breemen, Richard B. Van,Pezzuto, John M.,Fong, Harry H.S.,Chen, Ye-Gao,Zhang, Hong-Jie
, p. 641 - 647 (2010)
Kaempferol glycosides, named palmatosides A (1), B (2) and C (3), together with three known kaempferol glycosides, multiflorins A (4) and B (5), and afzelin (6), were isolated from the roots of the fern Neocheiropteris palmatopedata. Palmatosides A (1) and B (2) each possessed an unusual sugar moiety containing a 4,4-dimethyl-3-oxo-butoxy substituent group. The isolated compounds were evaluated for their cancer chemopreventive potential based on their ability to inhibit tumor necrosis factor alpha (TNF-α)-induced NF-κB activity, nitric oxide (NO) production, aromatase, quinone reductase 2 (QR2) and COX-1/-2 activities. Palmatosides B (2) and C (3) inhibited TNF-α-induced NF-κB activity with IC50 values of 15.7 and 24.1 μM, respectively; multiflorin A (4) inhibited aromatase enzyme with an IC50 value of 15.5 μM; afzelin (6) showed 68.3% inhibition against QR2 at a concentration of 11.5 μg/ml; palmatoside A (1) showed 52% inhibition against COX-1 enzyme at a concentration of 10 μg/ml; and multiflorin B (5) showed 52% inhibition against nitric oxide production at a concentration of 20 μg/ml. In addition, compounds 3-6 were shown to bind QR2 enzyme using LC-MS ultrafiltration binding assay.
Platinum-Catalyzed aqueous-Phase hydrogenation of d?Glucose to d?Sorbitol
Zhang, Xingguang,Durndell, Lee J.,Isaacs, Mark A.,Parlett, Christopher M.A.,Lee, Adam F.,Wilson, Karen
, p. 7409 - 7417 (2016)
Aqueous-phase hydrogenation of D-glucose to D-sorbitol was systematically investigated over silica-supported Pt nanoparticles to elucidate structure?reactivity relations and mechanistic insight. D-Glucose hydrogenation over large Pt particles competes with its isomerization to D-fructose over low-coordination (electron-deficient) Pt sites; D-sorbitol production by the former process was structure insensitive for nanoparticles spanning 3?17 nm, whereas isomerization was favored by smaller particles, with both pathways independent of the choice of fumed silica or mesoporous SBA-15 support. While D-fructose was readily hydrogenated to D-mannitol under the same reaction conditions, the latter underwent minimal isomerization to D-sorbitol, which is, therefore, a direct product of D-glucose ring opening and subsequent hydrogenation of the aldose conformer. D-Sorbitol production was favored by low D-glucose concentrations (<10 wt %), high H2 pressures (>40 bar), and low reaction temperatures (<140 °C), which suppressed undesired polymerization side reactions.
Microchip Device for Performing Enzyme Assays
Hadd, Andrew G.,Raymond, Daniel E.,Halliwell, John W.,Jacobson, Stephen C.,Ramsey, J. Michael
, p. 3407 - 3412 (1997)
An automated enzyme assay was performed within a microfabricated channel network. Precise concentrations of substrate, enzyme, and inhibitor were mixed in nanoliter volumes using electrokinetic flow. Reagent dilution and mixing were controlled by regulating the applied potential at me terminus of each channel, using voltages derived from an equivalent circuit model of the microchip. The enzyme β-galactosidase (β-Gal) was assayed using resorufin β-D-galactopyranoside (RBG), a substrate that is hydrolyzed to resorufin, a fluorescent product Reaction kinetics were obtained by varying the concentration of substrate on-chip and monitoring the production of resorufin using laser-induced fluorescence. Derived Michaelis-Menten constants compared well between an on-chip and a conventional enzyme assay. Bias in the derived Km and kcat was primarily due to the limited solubility of RBG and the associated lack of measurements at substrate concentrations exceeding the Km. A Ki of 8 μM for the inhibitor phenylethyl β-D-thiogalactoside (PETG) was determined from plots of initial rate versus substrate concentration obtained at three concentrations of PETG. The relative inhibition of β-Gal by lactose, p-hydroxymercuribenzoic acid, and PETG was determined by varying the inhibitor concentration with constant enzyme and substrate concentration. An enzyme assay performed on the microchip within a 20-min period required only 120 pg of enzyme and 7.5 ng of substrate, reducing the amount of reagent consumed by 4 orders of magnitude over a conventional assay.
A new steroidal saponin from Allium ampeloprasum var. porrum with antiinflammatory and gastroprotective effects
Adao, Camila Rodrigues,Da Silva, Bernadete Pereira,Parente, Jose Paz
, p. 306 - 310 (2011)
A new steroidal saponin was isolated from the bulbs of Allium ampeloprasum var. porrum. On the basis of chemical conversions and detailed analyses of 1H and 13C NMR spectra including 2D NMR spectroscopic techniques, its structure was established as 3-[(O-β-d-glucopyranosyl-(1 → 3)-β-d-glucopyranosyl-(1 → 2)-O-[O-β-d-glucopyranosyl-(1 → 3)]-O-β-d-glucopyranosyl-(1 → 4)-β-d-galactopyranosyl)oxy] -2,6-dihydroxy-(2α,3β,5α,6β,25R)-spirostane. Results of the present study indicated that the steroidal saponin showed haemolytic effects in the in vitro assays and demonstrated antiinflammatory activity and gastroprotective property using in vivo models.
New monoterpenoids from the stigmas of Crocus sativus
Fang, Qing-Wei,Fu, Wen-Wei,Yang, Jin-Ling,Lu, Yue,Chen, Jiang-Cheng,Wu, Pei-Ying,Zhang, Xue,Xu, Hong-Xi
, p. 102 - 109 (2022)
One new compound, crocusatin M (1), and three new glycosidic compounds, crocusatins N-P (2–4), along with nine known compounds were isolated from the dried stigmas of Crocus sativus. The structures of new compounds were elucidated on the basis of spectros
The main factors affecting the catalytic properties of Ru/Cs-HPA systems in one-pot hydrolysis-hydrogenation of cellulose to sorbitol
Gerasimov, Evgeniy Yu.,Gromov, Nikolay V.,Kozhevnikov, Ivan V.,Medvedeva, Tatiana B.,Panchenko, Valentina N.,Parmon, Valentin N.,Said-Aizpuru, Olivier,Taran, Oxana P.,Timofeeva, Maria N.
, (2020)
One-pot conversion of mechanically activated cellulose to sorbitol was investigated over bifunctional catalysts based on Ru (0.6, 1 and 3 wt.%) and cesium salts of heteropoly acids (HPA) Cs2.1H0.9PW12O40 and Cs3HSiW12O40 (Cs-PW and Cs-SiW, respectively). The maximal yield of sorbitol equal to 59 % and selectivity 94 % were achieved over the 1%Ru/Cs3HSiW12O40 catalyst. Physicochemical and catalytic data showed that the rate-determining step, i.e. the hydrolysis of cellulose, depended on the surface acidity of catalysts, whereas Ru content in catalyst affected both the hydrolysis and the hydrogenation steps. The kinetic parameters for one-pot conversion of cellulose were determined by mathematical modeling approach and were successfully used for the prediction of the yields of sorbitol and mannitol.
Two New Triterpenoid Glycosides from the Roots of Rosa cymosa Tratt.
Ma, Guo-Xu,Huang, Xiao-Yan,Dai, Hua-Nian,Zhong, Xiao-Qing,Zhou, Yan-Lin,Su, Zuo-Lin,He, Ying-Zi,Yang, Jun-Shan,Yuan, Jing-Quan,Xu, Xu-Dong
, p. 482 - 486 (2016)
Two new triterpenoid glycosides, 3α,19α,23α-trihydroxy-2-oxo-12-ursen-28-O-β-d-glucopyranoside (1) and 3α,19α,23α-trihydroxy-2-oxoolean-12-en-28-O-β-d-glucopyranoside (2) as well as three known compounds, 2α,3α,19α-trihydroxyolean-12-en-28-O-β-d-glucopyranoside (3), 2α,3α,19α,23-tetrahydroxy-12-ursen-28-O-β-d-glucopyranoside (4), and 2α,3β,19α,23-tetrahydroxyurs-12-en-28-oic acid (5) were isolated from 75% EtOH extract of Rosa cymosa. Their structures were elucidated by extensive spectroscopic methods. All the isolated compounds displayed moderate inhibitory activity against LPS-induced NO production in macrophages.
A new diterpenoid glucoside and two new diterpenoids from the fruit of Vitex agnus-castus
Ono, Masateru,Eguchi, Keisuke,Konoshita, Masatarou,Furusawa, Chisato,Sakamoto, Junich,Yasuda, Shin,Ikeda, Tsuyoshi,Okawa, Masafumi,Kinjo, Junei,Yoshimitsu, Hitoshi,Nohara, Toshihiro
, p. 392 - 396 (2011)
A new labdane-type diterpenoid glucoside and two new labdane-type diterpenoids were isolated from the fruit (chasteberry) of Vitex agnus-castus L. (Verbenaceae) along with 14 known compounds comprising seven labdane-type diterpenoids, one halimane-type diterpenoid, two oleanane-type triterpenoids, two ursane-type triterpenoids, one aromadendrane-type sesquiterpenoid, and one flavonoid. Their structures were characterized on the basis of spectroscopic data as well as chemical evidence. Furthermore, the antioxidative activities of the flavonoid were evaluated using five different analyses.
Anti-inflammatory constituents from Psychotria prainii H. Lév
Tran, Phi Hung,Le, Viet Dung,Do, Thi Ha,Nguyen, Thi Luyen,Nguyen, Phuong Thao,Nguyen, Trong Thong,Nguyen, Tien Dat
, p. 695 - 700 (2019)
One new and three known compounds were isolated from the ethanol extract of Psychotria prainii aerial parts. By means of spectroscopic methods, their structures were elucidated to be deacetylasperulosidic acid 6-ethyl ether (1), asperulosidic acid (2), asperuloside (3) and obtucarbamates C (4). The isolated compounds were evaluated for their inhibitory effect on NO production in LPS-stimulated RAW264.7 cells. Among them, compounds 2 and 4 exhibited strong effect with the IC50 values of 5.75?±?0.85 and 6.92?±?0.43?μM, respectively. This is the first report for the chemical composition and biological activity of P. prainii.
Effective conversion of cellobiose and glucose to sorbitol using non-noble bimetallic NiCo/HZSM-5 catalyst
Zada, Bakht,Yan, Long,Fu, Yao
, p. 1167 - 1174 (2018)
The tandem hydrolysis and hydrogenation of saccharides into sorbitol is an especially attractive reaction in the conversion of biomass. Here, an economical and efficient bimetallic catalyst for the transformation of glucose and cellobiose into sorbitol is reported. Non-precious metal based catalysts such as NiCo, Ni, and Co, were prepared via modified impregnation method, and NiCo/HZSM-5 showed superior performance for the synthesis of sorbitol (86.9% from cellobiose, 98.6% from D-glucose). Various characterizations, such as Brunner-Emmet-Teler (BET), X-ray diffraction (XRD), transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS), confirmed that NiCo alloy formed and highly dispersed in NiCo/HZSM-5 catalyst. The high performance of fabricated catalyst would be attributed to the formation of nickel-cobalt alloy over HZSM-5 zeolite surface. High temperature and H2 pressure were favorable for the tandem hydrolysis and hydrogenation reaction. Besides, the reaction pathway was also proposed based on the kinetics study. Cellobitol was detected as the intermediate in the reaction mixture. Furthermore, in the catalytic stability study, it was found that active metal species of NiCo/HZSM-5 were stable. The deactivation of catalyst would be due to the covering of acidic sites over NiCo/HZSM-5.
Antibacterial and cytotoxic phenolic metabolites from the fruits of amorpha fruticosa
Muharini, Rini,Diaz, Adriana,Ebrahim, Weaam,Mándi, Attila,Kurtán, Tibor,Rehberg, Nidja,Kalscheuer, Rainer,Hartmann, Rudolf,Orfali, Raha S.,Lin, Wenhan,Liu, Zhen,Proksch, Peter
, p. 169 - 180 (2017)
Fourteen new natural products, namely, 2-[(Z)- styryl]-5-geranylresorcin-1-carboxylic acid (1), amorfrutin D (2), 4-O-demethylamorfrutin D (3), 8-geranyl-3,5,7- trihydroxyflavanone (4), 8-geranyl-5,7,3′-trihydroxy-4′-methoxyisoflavone (5), 6-geranyl-5,7,3
Four new triterpenoids from callicarpa kwangtungensis
Zhou, Guo-Ping,Yu, Yan,Yuan, Ming-Ming,Ji, Tengfei,Fu, Hui-Zheng,Zhong, Rui-Jian
, p. 9071 - 9083 (2015)
Four new triterpenoids which were identifed as 2α,3β,6β,19α-Tetrahydroxyoleanolic acid 28-O-β-D-glucopyranoside (1), 2-O-β-D-glucopyranosyloxy-3α,19α-dihydroxyoleanolic acid (2), 2-O-β-D-glucopyranosyloxy-3α,19α-dihydroxyursolic acid (3), 2α,3α,6β,19α-Tetrahydroxyursolic acid 28-O-β-D-glucopyranoside (4), were isolated from the aerial parts of Callicarpa kwangtungensis together with three known triterpenoids identified as 2α,3β,21β-Trihydroxyursolic acid 28-O-β-D-glucopyranoside (5), 2α,3α,19α,23-tetrahydroxyoleanolic acid 28-O-β-D-glucopyranoside (6), 2α,3α,19α,23-Tetrahydroxyursolic acid 28-O-β-D-glucopyranoside (7). Their structures were elucidated by the combination of mass spectrometry (MS), one and two-dimensional NMR experiments.
Steric course of the hydrolysis of alpha,alpha-trehalose and alpha-D-glucosyl fluoride catalyzed by pig kidney trehalase.
Nakano,Brewer,Kasumi,Hehre
, p. 139 - 144 (1989)
We are unable to confirm the report of Labat et al.3 that pig kidney trehalase hydrolyzes alpha,alpha-trehalose to form solely alpha-D-glucose. Highly purified trehalase from pig renal cortex was found, in reactions monitored by 1H-n.m.r. spectra, to hydrolyze alpha,alpha-trehalose with the formation of both alpha- and beta-D-glucose. That the beta anomer constitutes the enzymically mobilized glucosyl residue is indicated by the further finding that beta-D-glucose is the product formed on hydrolysis of alpha-D-glucosyl fluoride by the enzyme. Present results show the stereochemical behavior of pig kidney trehalase in hydrolyzing alpha,alpha-trehalose to be indistinguishable from that reported by ourselves and others for trehalase preparations from a range of biological sources including rabbit renal cortex.
A new dihydrochalcone glycoside from the stems of Homalium stenophyllum
Wu, Shou-Yuan,Fu, Yan-Hui,Zhou, Qi,Bai, Meng,Chen, Guang-Ying,Han, Chang-Ri,Song, Xiao-Ping
, p. 953 - 958 (2018)
A new dihydrochalcone glycoside, phloretin-4-O-β-D-glucopyranoside (1), together with seven known flavonoids (2–8), were isolated from the stems of Homalium stenophyllum. The structure of 1 was elucidated by extensive spectroscopic methods and the known compounds were identified by comparisons with data reported in the literature. The known compounds (2–8) were isolated from the genus Homalium for the first time. All compounds were evaluated for their antibacterial activities against six pathogenic bacteria in vitro.
A new alkylene dihydrofuran glycoside with antioxidation activity from the root bark of Morus alba L
Fu, Wei,Lei, Yong Fang,Cai, Ya Ling,Zhou, Dao Nian,Ruan, Jin Lan
, p. 821 - 823 (2010)
A new alkylene dihydrofuran glycoside (1) was isolated from the root bark of Morus alba L., along with moracin M-3′-O-β-Dglucopyranoside (2), and moracin M-6, 3′-di-O-β-D-glucopyranoside (3). Compound 1 was identified as 2-methylene-3-methoxy-2, 5-dihydrofuran-4-O-β-D- glucopyranoside on the basis of chemical and spectroscopic data including 1D and 2D NMR spectral analysis. In addition, the antioxidant activity of 1 was evaluated using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2′-azinobis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS) assay. The IC50 values were 2.49 and 0.45 mg/mL, respectively.
Three new phthalides from gnaphalium adnatum
Zheng, Xing-Ping,Cui, Qiong-Fang,Zhao, Jing-Feng,Yang, Li-Juan,Zhang, Hong-Bin,Yang, Xiao-Dong,Li, Liang
, p. 1638 - 1643 (2014)
Three new phthalides, gnaphalides A-C (1 - 3, resp.), together with three known phthalides, were isolated from the aerial part of Gnaphalium adnatum. The structures of the new compounds were elucidated as 6-(1,1-dimethylprop-2-en-1-yl)-5,7-dihydroxy-2-benzofuran-1(3H)-one (1), 5-hydroxy-7- [(2-hydroxy-3-methylbut-3-en-1-yl)oxy]-2-benzofuran-1(3H)-one (2), and 1,3-dihydro-7-[(3-methylbut- 2-en-1-yl)oxy]-1-oxo-2-benzofuran-5-yl β-d-glucopyranoside (3) on the basis of spectral analyses. The structure of 1 was also confirmed by X-ray crystallographic analysis. The three known phthalides, identified as 5,7-dihydroxyisobenzofuran-1(3H)-one (4), anaphatol (5), and 7-O-(β-glucopyranosyl)-5- hydroxyisobenzofuran-1(3H)-one (6), were isolated from the genus Gnaphalium for the first time.
IMMOBILIZED ENZYME KINETIC STUDY OF D-GLUCOSE MUTAROTATION BY FLOW INJECTION ANALYSIS.
Stults,Wade,Crouch
, p. 2245 - 2247 (1987)
A novel analytical application of immobilized enzymes is presented in which the mutarotation kinetics of D-glucose are studied. A glucose oxidase single bead string reactor is combined with the Trinder reaction in a flow injection system. The apparatus is used to monitor the mutarotation of D-glucose in a batch reactor. The mutarotation coefficients obtained are in good agreement with the literature values. Phosphate ion is shown to catalyze the reaction and the mutarotation coefficient is directly proportional to its concentration. The selectivity of the enzyme reactor is also demonstrated.
An anti-inflammatory C-stiryl iridoid from Camptosorus sibiricus Rupr.
Wang, Fang,Jia, Qing-Wen,Yuan, Zhen-Hai,Lv, Ling-Yue,Li, Min,Jiang, Zhi-Bo,Liang, Da-Lian,Zhang, Dai-Zhou
, p. 378 - 381 (2019)
A new iridoid glycoside, named camptoside (1), together with three known compounds as dehydrodiconiferyl alcohol-9′-O-β-D-glucopyranoside (2), aesculetin (3) and vajicoside (4), have been isolated from Camptosorus sibiricus Rupr. (Aspleniaceae). Their structures were established on the basis of spectroscopic analysis, especially 1D- and 2D-NMR data, and by comparison of their spectroscopic and physical data with those reported in the literature. Compounds 1–3 exhibited inhibitions of nitric oxide production in lipopolysaccharide-induced RAW 264.7 macrophages with IC50 values of 11.2, 8.3 and 9.4 μM, respectively.
A new 2-(2-phenylethyl)chromone glycoside in Chinese agarwood “Qi-Nan” from Aquilaria sinensis
Shao, Hang,Mei, Wen-Li,Kong, Fan-Dong,Dong, Wen-Hua,Li, Wei,Zhu, Guo-Peng,Dai, Hao-Fu
, p. 42 - 46 (2017)
A new 2-(2-phenylethyl)chromone glycoside, 2-[2-(4-glucosyloxy-3-methoxyphenyl)ethyl]chromone (1), was isolated from the high-quality Chinese agarwood “Qi-Nan” originating from Aquilaria sinensis (Lour.) Glig. The structure including the absolute configuration of the sugar moiety was elucidated by spectroscopic techniques (UV, IR, 1D and 2D NMR), MS analysis, PMP-labeling HPLC analysis methods, as well as comparison with literature data. To the best of our knowledge, it is the first time that chromone glycoside was discovered in agarwood, or even in the whole Aquilaria plants.
Microtropiosides A-F: ent-Labdane diterpenoid glucosides from the leaves of Microtropis japonica (Celastraceae)
Koyama, Yuka,Matsunami, Katsuyoshi,Otsuka, Hideaki,Shinzato, Takakazu,Takeda, Yoshio
, p. 675 - 681 (2010)
From a 1-BuOH-soluble fraction of a MeOH extract of the leaves of Microtropis japonica, collected in the Okinawa islands, six ent-labdane glucosides, named microtropiosides A-F, were isolated together with one known acyclic sesquiterpene glucoside. Their structures were elucidated by a combination of spectroscopic analyses, and their absolute configurations determined by application of the β-d-glucopyranosylation-induced shift-trend rule in 13C NMR spectroscopy and the modified Mosher's method.
Chemical constituents from the fruit of Gardenia jasminoides and their inhibitory effects on nitric oxide production
Peng, Kaifeng,Yang, Liguo,Zhao, Shizhe,Chen, Lixia,Zhao, Feng,Qiu, Feng
, p. 1127 - 1131 (2013)
Three new iridoid glycosides, 6″-O-trans-caffeoylgenipin gentiobioside (1), genipin 1-O-β-d-apiofuranosyl (1→6)-β-d- glucopyranoside (2), genipin 1-O-α-d-xylopyranosyl (1→6)-β-d- glucopyranoside (3), three new monocyclic monoterpenoids, jasminoside R (4),
Three new iridoid glycosides from the fruit of gardenia jasminoides var. radicans
Qin, Fang-Min,Meng, Ling-Jie,Zou, Hui-Liang,Zhou, Guang-Xiong
, p. 1071 - 1074 (2013)
Three new iridoid glycosides, 6″-O-trans-feruloylgenipin gentiobioside (1), 2'-O-trans-p-coumaroylgardoside (2), 2'-O-trans- feruloylgardoside (3), were isolated from the fruit of Gardenia jasminoides var. radicans MAKINO (Rubiaceae). The structures of th
A new cerebroside from the entomopathogenic fungus Ophiocordyceps longiissima: Structural-electronic and antioxidant relations. Experimental and DFT calculated studies
Elshamy, Abdelsamed I.,Yoneyama, Tatsuro,Trang, Nguyen Van,Son, Ninh The,Okamoto, Yasuko,Ban, Sayaka,Noji, Masaaki,Umeyama, Akemi
, (2020)
The chemical characterization of n-butanol soluble extract of the fungus Ophiocordyceps longiissima NBRC 106965 afforded a new cerebroside, 1-O-(β-d-glucopyranosyl)-(2S,3R,4E,8E)-2-[(2′R)-2′-hydroxyheptadecanoylamino]-9-methylheptadeca-4,8-diene-1,3-diol, trivially named ophiocordylongiiside A (1), in addition to 22E,24R-24-methylcholest-5,22-diene-3-O-β-D-O-glucopyranoside (2). The chemical structure of 1 was established based upon the by modern spectroscopic and chemical techniques. Compound 1 exhibited strong DPPH-free radical scavenging with the IC50 value of 55.72 mg/mL. The biological result can be explained by density functional theoretical (DFT) calculation, in which antioxidant activity is mostly controlled by the O–H bond dissociation enthalpy (BDE) following hydrogen atom transfer (HAT) mechanism. In all studied environments, the lowest BDE values are found in 2′-OH.
Diterpenoids with immunosuppressive activities from cinnamomum cassia
Zeng, Junfen,Xue, Yongbo,Shu, Penghua,Qian, Huiqin,Sa, Rongjian,Xiang, Ming,Li, Xiao-Nian,Luo, Zengwei,Yao, Guangmin,Zhang, Yonghui
, p. 1948 - 1954 (2014)
Three new diterpenoids with unprecedented carbon skeletons, cinncassiols F (1) and G (2) and 16-O-β-d-glucopyranosyl-19-deoxycinncassiol G (3), a new isoryanodane diterpenoid, 18-hydroxyperseanol (4), six known isoryanodane diterpenoids, 5-10, and a known ryanodane diterpenoid, 11, were isolated from the stem bark of Cinnamomum cassia. Compound 1 possesses an 11,13:12,13-diepoxy- 6,11-epoxy:12,13-disecoisoryanodane diterpenoid skeleton bearing ketal and hemiketal functionalities, whereas compounds 2 and 3 feature an 11,12-secoisoryanodane diterpenoid skeleton with an 11,6-lactone moiety. The structures of the four new diterpenoids, 1-4, and their absolute configurations were established using HRESIMS, NMR, ECD, single-crystal X-ray diffraction, and chemical methods. Compounds 2 and 11 significantly inhibited the proliferation of murine T cells induced by ConA.
Exopolysaccharide Produced by Probiotic Bacillus albus DM-15 Isolated From Ayurvedic Fermented Dasamoolarishta: Characterization, Antioxidant, and Anticancer Activities
Kalimuthu, Palanisamy,Ma, Yongkun,Mathivanan, Krishnamurthy,Rai, Amit Kumar,Saravanan, Kandasamy,Sathiyanarayanan, Ganesan,Sekar, Soundarapandian,Sudharsan, Kumaresan,Vinothkanna, Annadurai
, (2022/03/31)
An exopolysaccharide (EPS) was purified from the probiotic bacterium Bacillus albus DM-15, isolated from the Indian Ayurvedic traditional medicine Dasamoolarishta. Gas chromatography-mass spectrophotometry and nuclear magnetic resonance (NMR) analyses revealed the heteropolymeric nature of the purified EPS with monosaccharide units of glucose, galactose, xylose, and rhamnose. Size-exclusion chromatography had shown the molecular weight of the purified EPS as around 240 kDa. X-ray powder diffraction analysis confirmed the non-crystalline amorphous nature of the EPS. Furthermore, the purified EPS showed the maximum flocculation activity (72.80%) with kaolin clay and emulsification activity (67.04%) with xylene. In addition, the EPS exhibits significant antioxidant activities on DPPH (58.17 ± 0.054%), ABTS (70.47 ± 0.854%) and nitric oxide (58.92 ± 0.744%) radicals in a concentration-dependent way. Moreover, the EPS showed promising cytotoxic activity (20 ± 0.97 μg mL–1) against the lung carcinoma cells (A549), and subsequent cellular staining revealed apoptotic necrotic characters in damaged A549 cells. The EPS purified from the probiotic strain B. albus DM-15 can be further studied and exploited as a potential carbohydrate polymer in food, cosmetic, pharmaceutical, and biomedical applications.
Three new steroidal saponins from the roots and rhizomes of Rohdea chinensis (Baker) N.Tanaka (synonym Tupistra chinensis Baker)
Song, Bei,Huang, Wenli,Li, Yuze,Zhang, Yangyang,Zhang, Huawei,Jiang, Yi,Deng, Chong,Song, Xiaomei,Liu, Jianli
, p. 108 - 115 (2019/06/10)
Three new steroidal saponins (1–3), together with four known compounds (4–7), were isolated from the roots and rhizomes of Rohdea chinensis Baker, and their structures were determined as (24S, 25 R)-1β-hydroxy-3β-[(β-D-glucopyranoside)oxy]-spirost-5-en-24-yl-β-D-glucopyranoside (1) and (24S)-spirost-25(27)-en-1β, 3β, 4β, 5β, 6β, 24β-hexahydroxy-24-O-β-D-glucopyranoside (2), (22S, 25S)-1β, 3β, 4β, 5β, 26, 27-hexanol-furospirost-5, 26-O-β-D-glucopyranoside (3), together with four known compounds 3-epi-diosgenin-3-β-D-glucopyranosid (4), 3-epiruscogenin (5), 25(R)-1β-hydroxy-spirost-5-en-3α-yl-O-β-D-glucopyranoside (6) and tupichinin A (7), on the basis of physico-chemical properties and spectral analysis. In this study, compounds 1–3 and 5–7 were evaluated for their cytotoxic activity against SW620, A549 and HepG2 tumor cell lines. Among them, compound 7 showed moderate cytotoxicity against two human cancer cell lines A549 and HepG 2 with IC50 values of 25.3 ± 2.6 and 26.1 ± 2.5 μM, respectively.
Discovery of New Iridoids as Farnesoid X Receptor Agonists from Morinda officinalis: Agonistic Potentials and Molecular Stimulation
Luan, Zhi-Lin,Qiao, Fei,Zhao, Wen-Yu,Ming, Wen-Hua,Yu, Zhen-Long,Liu, Jie,Dai, Sheng-Yun,Jiang, Shuang-Hui,Lian, Chao-Jie,Sun, Cheng-Peng,Zhang, Bao-Jing,Zheng, Jian,Ma, Shuang-Cheng,Ma, Xiao-Chi
, p. 1288 - 1296 (2021/05/06)
The investigation of Morinda officinalis led to the isolation of twelve compounds (1—12), including three new iridoid glycosides morindalins A—C (1—3) and nine known compounds (4—12). Their structural identifications were conducted using HRMS, 1D and 2D NMR, and electronic circular dichroism (ECD) spectra as well as quantum chemical computations. Compound 6 displayed the most significantly agonistic activity against farnesoid X receptor (FXR) with an EC50 value of 7.18 μM, and its agonistic effect was verified through the investigation of FXR downstream target genes including small heterodimer partner 1 (SHP1), bile salt export pump (BSEP), and organic solute transporter subunit alpha and beta (OSTα and OSTβ). The potential interaction of compound 6 with FXR was analyzed by molecular docking and molecular dynamics stimulation, revealing that amino acid residues Leu287, Thr288, and Ser332 played a crucial role in the activation of compound 6 towards FXR. These findings suggested that compound 6 could be regarded as a potential candidate for the development of FXR agonists.