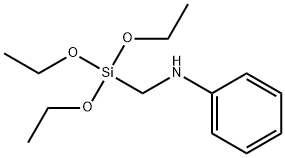
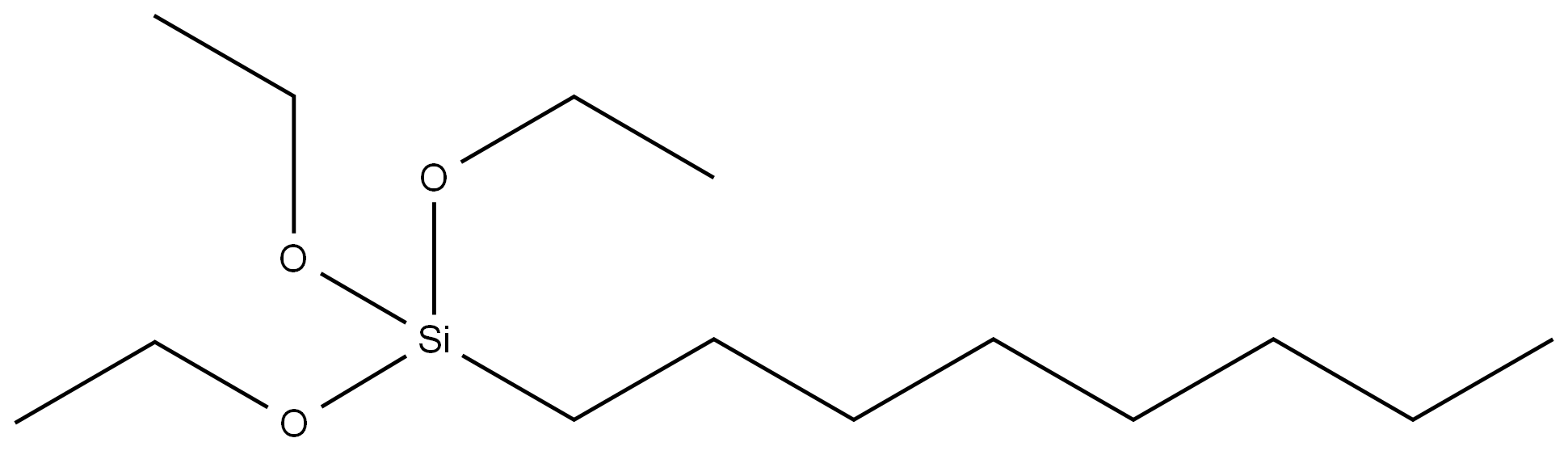
Anilino-methyl-triethoxysilane literature
Linear Hydroaminoalkylation Products from Alkyl-Substituted Alkenes
Warsitz, Michael,Doye, Sven
supporting information, p. 15121 - 15125 (2020/10/23)
The regioselective conversion of alkyl-substituted alkenes into linear hydroaminoalkylation products represents a strongly desirable synthetic transformation. In particular, such conversions of N-methylamine derivatives are of great scientific interest, because they would give direct access to important amines with unbranched alkyl chains. Herein, we present a new one-pot procedure that includes an initial alkene hydroaminoalkylation with an α-silylated amine substrate and a subsequent protodesilylation reaction that delivers linear hydroaminoalkylation products with high selectivity from simple alkyl-substituted alkenes. For that purpose, new titanium catalysts have been developed, which are able to activate the α-C?H bond of more challenging α-silylated amine substrates. In addition, a direct relationship between the ligand structure of the new catalysts and the obtained regioselectivity is described.
METHOD FOR PRODUCING AMINO-ORGANOSILANES
-
Page/Page column 5, (2011/07/29)
In the preparation of aminoorganylsilanes and cyclic aminosilanes by reaction of an organyl amine with a haloorganylsilane, the byproduct halide salt of the amine reactant is decomposed to amine by addition of a base whose halide salt forms a liquid phase at a temperature below 200° C., and the liquid base halide is separated from the reaction mixture.
Facile cleavage of Si-C bonds during the sol-gel hydrolysis of aminomethyltrialkoxysilanes - A new method for the methylation of primary amines
Adima, Augustin,Bied, Catherine,Moreau, Joel J. E.,Man, Michel Wong Chi
, p. 2582 - 2588 (2007/10/03)
The reaction of chloromethyltriethoxysilane with (1R,2R)-bis(methylamino) cyclohexane (1) afforded the corresponding bis-silylated compound 2. The sol-gel hydrolysis of 2 did not give the expected bridged silsesquioxane owing to quantitative Si-C-bond cleavage. Instead, silica and (1R,2R)-bis(dimethylamino) cyclohexane (3) were obtained. This reaction was exploited to propose a new route for the methylation of amines. Such methylation reaction of amines could be extended to other amines and provides a new method for the selective monomethylation of primary amines. Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, Germany, 2004.