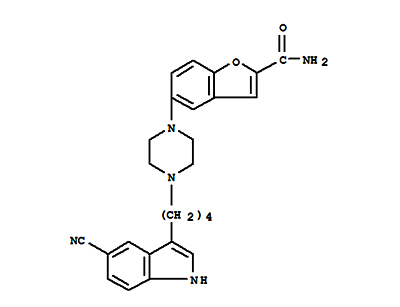
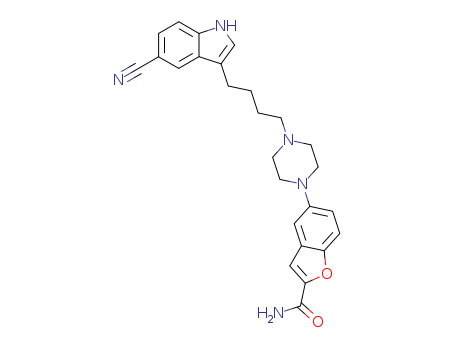
VILAZODONE literature
Preparation method of vilazodone
-
Paragraph 0033-0052, (2021/02/06)
The invention relates to a preparation method of vilazodone. The preparation method comprises the steps of by using a compound 1 and a compound 2 as raw materials, carrying out one-step reaction underthe condition that trifluoroacetic acid is used as a solvent to obtain a compound I, namely the vilazodone. Compared with the prior art, the method has the advantages of short reaction route, easy post-treatment, high yield, cheap and easily available reagents used in the reaction, convenient operation, simple post-treatment, low cost, small environmental pollution, and suitableness for industrial production of vilazodone.
An investigation of the synthesis of vilazodone
Hu, Fan,Su, Weike
, p. 243 - 247 (2020/01/08)
A novel synthetic route toward vilazodone is described by using 4-cyanoaniline and 5-bromo-2-hydroxybenzaldehyde as starting materials, with an overall yield of 24% and 99% purity. First, the intermediate (3-(4-chlorobutyl)-1H-indole-5-carbonitrile) is synthesized via diazotization of 4-cyanoaniline, followed by Fischer indole cyclization with 6-chlorohexanal. Subsequently, another intermediate, 5-(piperazin-1-yl)benzofuran-2-carboxamide, is generated via aromatic nucleophilic substitution of 5-bromobenzofuran-2-carboxamide with piperazine. Finally, vilazodone is obtained via nucleophilic substitution of the above two key intermediates by treatment with Et3N/K2CO3. In comparison to the original process, this route avoids the use of expensive and toxic reagents and resolves issues such as safety, environmental concerns, and high costs.
Preparation method of vilazodone hydrochloride
-
Page/Page column 8-11, (2019/02/04)
The invention discloses a preparation method of vilazodone hydrochloride. The preparation method comprises the following steps: (1) in the presence of ammonium hydroxide or ammonium hydroxide and N-methylpyrrolidone, enabling 5-(1-piperazinyl)-benzofuran-2-ethyl formate hydrochloride to be subjected to ammonolysis reaction to obtain 5-(1-piperazinyl)-benzofuran-2-formamide; (2) in the presence ofalkali, enabling the 5-(1-piperazinyl)-benzofuran-2-formamide and 3-(4-chlorobutyl) indole-5-formonitrile to be subjected to nucleophilic substitution reaction to obtain a crude product of vilazodone,wherein the alkali is mixed alkali of sodium iodide, N,N-diisopropylethylamine and triethylamine or 1,8-diazabicycloundec-7-ene; (3) refining the crude product of vilazodone to obtain a refined product of vilazodone; and (4) enabling the refined product of vilazodone to be subjected to salt-forming reaction to obtain the vilazodone hydrochloride. By virtue of the ammonolysis reaction and the nucleophilic substitution reaction, the yield is higher, and the purity is higher; and the yield of the vilazodone hydrochloride is increased.
Preparation method of vilazodone or hydrochloride thereof
-
, (2018/09/14)
The invention relates to a preparation method of an antidepressant drug vilazodone or a hydrochloride thereof, wherein high-purity and high-yield vilazodone is obtain through the crystallization of aN-methylpyrrolidone/water system. According to the present invention, the method overcomes the defects and the disadvantages of the existing vilazodone preparation method, is suitable for the industrial preparation of vilazodone hydrochloride, and has great positive progress effect and practical application value.