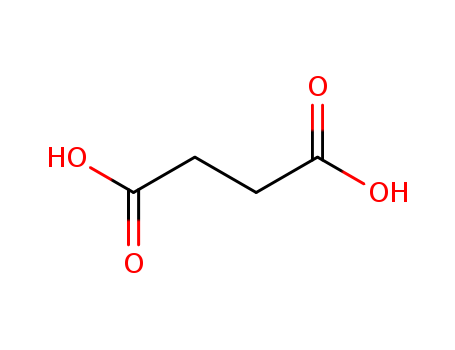
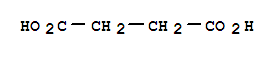Succinic acid literature
RHODIUM(III) COMPLEXES WITH O-ALKYL-S-ALKYL THIOCARBONATES AS CATALYSTS FOR THE HOMOGENEOUS HYDROGENATION OF UNSATURATED COMPOUNDS
Maistrenko, V. N.,Rusakov, I. A.,Bondareva, S. O.,Murinov, Yu. I.,Tolstikov, G. A.
, p. 2149 - 2151 (1989)
Catalytic activity was found for Rh3+ complexes with O-alkyl-S-alkyl thiocarbonates in the homogeneous hydrogenation of unsaturated compounds.Cyclic voltamperometry was used to detect the formation of rhodium hydride intermediates during the hydrogenation of alkenes in the presence of these complexes.
Catalytic oxidation of furan and hydrofuran compounds. 7. Production of 2(5H)-furanone by oxidation of furfural with hydrogen peroxide and some of its transformations in aqueous solutions
Badovskaya,Latashko,Poskonin,Grunskaya,Tyukhteneva,Rudakova,Pestunova,Sarkisyan
, p. 1040 - 1048 (2002)
Data on the synthesis of 2(5H)-furanone by the oxidation of furfural with aqueous hydrogen peroxide under the conditions of autocatalysis by the accumulating acids and also in the presence of catalytic amounts of Cr(VI) and Mo(VI) compounds are presented.
Boosting one-step conversion of cyclohexane to adipic acid by NO2 and VPO composite catalysts
Jian, Jian,You, Kuiyi,Duan, Xuezhi,Gao, Hongxu,Luo, Qing,Deng, Renjie,Liu, Pingle,Ai, Qiuhong,Luo, He'an
, p. 3320 - 3323 (2016)
We demonstrate VPO composites as efficient catalysts for highly selective oxidation of cyclohexane to adipic acid with NO2. In particular, the Ni-Al-VPO composite catalyst exhibits the striking conversion of cyclohexane (60.6%) and exceptionally high selectivity towards adipic acid (85.0%). Moreover, N2O is an environmentally harmful gas, and its yield in the present process is only 0.03 t/t adipic acid, which is far below that obtained using the industrial method (0.3 t/t adipic acid). This work provides a new strategy for the one-step synthesis of dicarboxylic acids from cycloalkanes.
Conjugated microporous polymers as a visible light driven platform for photo-redox conversion of biomass derived chemicals
Chen, Bo,Chen, Lang,Chen, Shanyong,Jin, Yongdong,Kang, Jinyang,Ma, Lijian,Xia, Chuanqin,Yan, Hongjian,Yan, Zijun
, p. 3607 - 3611 (2021)
Photocatalytic conversion of biomass derived chemicals to valuable products is a highly sustainable process. Herein we report the photocatalytic hydrogenation of maleic acid to succinic acid and oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran using the same conjugated porous polymers (CMPs). The CMPs were constructed from 2,4,6-(tri-2-thienyl)-1,3,5-triazine as the knots and different benzene derivatives as linkers, and their morphologies, redox potentials, charge separation efficiency, and the consequent photocatalytic performance have been controlled. As a result, the CMP with benzene as the linker features the highest photocatalytic activities with production rates of 4.66 mmol g?1h?1for succinic acid and 0.53 mmol g?1h?1for 2,5-diformylfuran, respectively. Most importantly, high photocatalytic activity has also been achieved under natural sunlight irradiation, implying its feasibility as an efficient photocatalytic platform for solar-to-chemical energy conversion.
-
Bruylants
, (1923)
-
Metal Sub-nanoclusters Confined within Hierarchical Porous Carbons with High Oxidation Activity
Zhao, Xin,Kong, Xiangpeng,Wang, Fengliang,Fang, Ruiqi,Li, Yingwei
, p. 10842 - 10849 (2021)
Metal sub-nanoclusters (SNCs) have shown great promise for a variety of catalytic reactions. However, the fabrication of stable metal SNCs simultaneously with high dispersion and high metal contents remains a challenge. Herein, we report a novel and versatile strategy for the synthesis of various bimetal SNCs stabilized within hierarchical porous carbons (HPC). This facile synthesis only involves the self-assembly of a metal-organic framework (MOF) as the precursor, a molten salt assisted pyrolysis process and the final metal replacement. The metal SNCs (mostly less than 0.8 nm) derived from the metal nodes of the MOF are exclusively confined and homogeneously dispersed throughout the organic ligands derived HPC at high loadings (up to 11.2 wt %). The obtained Cu-Pd@HPC composite exhibits superior catalytic activity and recycling durability in the selective transformation of furfural to maleic acid, achieving 97.8 % yield of maleic acid with a TOF value as high as 20.1 h?1 under mild conditions. DFT calculations reveal that the introduction of Pd shifts the partial density of states of Cu toward the Fermi level, leading to stronger chemisorption of furfural to enhance the catalytic activity.
Highly active and selective nickel-platinum catalyst for the low temperature hydrogenation of maleic anhydride to succinic anhydride and synthesis of succinic acid at 40 °c
Li, Jie,Tian, Wei-Ping,Shi, Li
, p. 565 - 571 (2011)
PtNi bimetallic and Ni monometallic catalysts supported on HY-Al 2O3, HX-Al2O3, ZSM-5-Al 2O3, USY-Al2O3, Beta-Al 2O3 and Al2O3 were prepared and evaluated for the hydrogenation of maleic anhydride in the temperature range of 40-150 °C. Results from flow reactor studies showed that supports strongly affected the catalytic properties of different bimetallic and monometallic catalysts. The results showed that the HY-Al2O3 support exhibited the highest activity and selectivity. Using NiPt/Al2O 3-HY catalyst and performing the reaction, it was possible to carry out the lowest reaction temperature ever carried at 100% conversion. Adding a small amount of Pt (0.5) to the Ni (5%)/Al2O3-HY catalyst that is effective for increasing the selectivity and activity. We also found that PtNi is an efficient catalyst for the one-pot conversion of maleic acid into succinic acid with 100% conversion at 40 °C. Graphical Abstract: Hydrogenation activity was found to correlate to the extent of PtNi bimetallic bond formation, as characterized by the analysis of XRD and TPR.[Figure not available: see fulltext.]
The contribution to kappa number from hexeneuronic acid groups in pulp xylan
Li, Jiebing,Gellerstedt, Goeran
, p. 213 - 218 (1997)
The kappa number of chemical pulps is widely used both in mill operation and in laboratory work as a measure of the degree of delignification in pulping, oxygen delignification, and prebleaching. Recently, it has been shown that the kappa number reflects not only lignin but also carbohydrate structures sensitive to oxidation by permanganate, notably hexeneuronic acid groups linked to xylan. In the present work, the kappa number units originating from hexeneuronic acid groups calculated on a molar basis have been determined in two different ways, viz. by permanganate oxidation of model compounds and by selective elimination of hexeneuronic acid groups from a series of kraft pulps. The results are in good agreement with each other and demonstrate that 10 μmol of hexeneuronic acid correspond to 0.84-0.86 kappa units. From kappa number determinations combined with hydrolysis of the pulp with mercuric acetate, it is possible to calculate the amount of hexeneuronic acid groups present in a pulp.
Near infrared activation of an anticancer PtIV complex by Tm-doped upconversion nanoparticles
Ruggiero, Emmanuel,Hernández-Gil, Javier,Mareque-Rivas, Juan C.,Salassa, Luca
, p. 2091 - 2094 (2015)
The PtIV complex cis,cis,trans-[Pt(NH3)2(Cl)2(O2CCH2CH2CO2H)2] is photoactivated by near infrared light (980 nm) using NaYF4:Yb3+/Tm3+@NaYF4 core-shell upconversion nanoparticles. Coupling of this cisplatin precursor with the biocompatible PEGylated phospholipid DSPE-PEG(2000)-NH2 affords a valuable approach to decorate the surface of the nanoparticles, providing novel photoactivatable nanomaterials capable of releasing PtII species upon NIR light excitation. This journal is
Encapsulation of Pt(IV) prodrugs within a Pt(II) cage for drug delivery
Zheng, Yao-Rong,Suntharalingam, Kogularamanan,Johnstone, Timothy C.,Lippard, Stephen J.
, p. 1189 - 1193 (2015)
This report presents a novel strategy that facilitates delivery of multiple, specific payloads of Pt(IV) prodrugs using a well-defined supramolecular system. This delivery system comprises a hexanuclear Pt(II) cage that can host four Pt(IV) prodrug guest molecules. Relying on host-guest interactions between adamantyl units tethered to the Pt(IV) molecules and the cage, four prodrugs could be encapsulated within one cage. This host-guest complex, exhibiting a diameter of about 3 nm, has been characterized by detailed NMR spectroscopic measurements. Owing to the high positive charge, this nanostructure exhibits high cellular uptake. Upon entering cells and reacting with biological reductants such as ascorbic acid, the host-guest complex releases cisplatin, which leads to cell cycle arrest and apoptosis. The fully assembled complex displays cytotoxicity comparable to that of cisplatin against a panel of human cancer cell lines, whereas the cage or the Pt(IV) guest alone exhibit lower cytotoxicity. These findings indicate the potential of utilising well-defined supramolecular constructs for the delivery of prodrug molecules.
Reducing Agent-Mediated Nonenzymatic Conversion of 2-Oxoglutarate to Succinate: Implications for Oxygenase Assays
Khan, Amjad,Schofield, Christopher J.,Claridge, Timothy D. W.
, p. 2898 - 2902 (2020)
l-Ascorbate (l-Asc) is often added to assays with isolated FeII- and 2-oxoglutarate (2OG)-dependent oxygenases to enhance activity. l-Asc is proposed to be important in catalysis by some 2OG oxygenases in vivo. We report observations on the nonenzymatic conversion of 2OG to succinate, which is mediated by hydrogen peroxide generated by the reaction of l-Asc and dioxygen. Slow nonenzymatic oxidation of 2OG to succinate occurs with some, but not all, other reducing agents commonly used in 2OG oxygenase assays. We intend these observations will help in the robust assignment of substrates and inhibitors for 2OG oxygenases.
Glutaric and Succinic Acids in the Cobalt Acetate Catalyzed Oxidation of Cyclohexane with Oxygen
Schulz, Johann G. D.,Onopchenko, Anatoli
, p. 3716 - 3719 (1980)
-
Hydroxylamine as an oxygen nucleophile. Chemical evidence from its reaction with a phosphate triester
Kirby, Anthony J.,Souza, Bruno S.,Medeiros, Michelle,Priebe, Jacks P.,Manfredi, Alex M.,Nome, Faruk
, p. 4428 - 4429 (2008)
The reaction of hydroxylamine with 2,4-dinitrophenyl diethyl phosphate gives the O-phosphorylated product, which is rapidly converted to hydrazine and nitrogen gas in the presence of the excess of hydroxylamine. The Royal Society of Chemistry.
-
Ts'ai
, p. 1886 (1951)
-
The effect of Br- and alkali in enhancing the oxidation of furfural to maleic acid with hydrogen peroxide
Yang, Tao,Li, Wenzhi,Ogunbiyi, Ajibola T.
, (2021)
This study was focused on investigating a novel catalytic system for the selective conversion of furfural to maleic acid (MA) in an aqueous system with hydrogen peroxide as an oxidant. A series of experiments that study the impacts of catalyst species, furfural concentration, catalyst dosage, reaction temperature, residue time, hydrogen peroxide concentration, excess water content, and solvent types on the oxidation of furfural to MA was carried out. The results showed that the co-existence of Br- and alkali sites might play a vital role in furfural oxidation, which could improve the MA yield remarkably. Under 90 °C for 3 h, 72.4 % MA yield was obtained with KOH and KBr as co-catalyst in an aqueous phase. Moreover, a possible reaction pathway of furfural oxidation was proposed on the basis of our reaction system.
Characterization and reactivity of Mn-Ce-O composites for catalytic ozonation of antipyrine
Xing, Shengtao,Lu, Xiaoyang,Ren, Limei,Ma, Zichuan
, p. 60279 - 60285 (2015)
Mn-Ce-O composites were prepared by a redox-precipitation method and used as a catalyst for ozonation of antipyrine (AP) in aqueous solution. The phase composition, surface area and electron transfer ability of the products are dependent on the molar ratio of precursors. Mn-Ce-O(8/2) exhibited the highest activity for the degradation and mineralization of AP with ozone, which can be attributed to its high electron transfer ability. The decomposition of ozone into .OH can be accelerated through the electron transfer between ozone and catalyst. The refractory intermediates in ozonation and catalytic ozonation were identified and the catalytic performances of Mn-Ce-O(8/2) for these intermediates were also investigated. The hydroxyl radical reaction played an important role in the degradation of the refractory intermediates, and the contribution of surface reactions was strengthened with decreasing pH. A possible mechanism for the catalytic ozonation of AP was proposed.
Photoelectroreduction of Building-Block Chemicals
Chen, Fengjiao,Cui, Wei,Zhang, Jie,Wang, Yeyun,Zhou, Junhua,Hu, Yongpan,Li, Yanguang,Lee, Shuit-Tong
, p. 7181 - 7185 (2017)
Conventional photoelectrochemical cells utilize solar energy to drive the chemical conversion of water or CO2 into useful chemical fuels. Such processes are confronted with general challenges, including the low intrinsic activities and inconvenient storage and transportation of their gaseous products. A photoelectrochemical approach is proposed to drive the reductive production of industrial building-block chemicals and demonstrate that succinic acid and glyoxylic acid can be readily synthesized on Si nanowire array photocathodes free of any cocatalyst and at room temperature. These photocathodes exhibit a positive onset potential, large saturation photocurrent density, high reaction selectivity, and excellent operation durability. They capitalize on the large photovoltage generated from the semiconductor/electrolyte junction to partially offset the required external bias, and thereby make this photoelectrosynthetic approach significantly more sustainable compared to traditional electrosynthesis.
A rationale of the BaeyerVilliger oxidation of cyclohexanone to ε-caprolactone with hydrogen peroxide: Unprecedented evidence for a radical mechanism controlling reactivity
Cavani, Fabrizio,Raabova, Katerina,Bigi, Franca,Quarantelli, Carla
, p. 12962 - 12969 (2010)
We demonstrate, for the first time, in the BaeyerVilliger oxidation of cyclohexanone with aqueous hydrogen peroxide under conditions aimed at obtaining ε-caprolactone, that a thermally activated radical reaction leads to the concurrent formation of adipic acid, even when a stoichiometric amount of the oxidant is used. In fact, ε-caprolactone is the primary reaction product, but it is more reactive than cyclohexanone, and quickly undergoes consecutive transformations. When titanium silicalite-1 (TS-1) is used as a catalyst, the high concentration of hydroxy radicals within its pores accelerates the reaction rates, and the consecutive formation of adipic acid (and of lighter diacids as well) becomes largely kinetically preferred. The proper choice of the solvent, which also may act as a radical scavenger, both without catalyst and with TS-1, is a powerful tool for controlling the rates of the various reactions involved.
Unusual conversion of 5-hydroxy-2(5H)-furanone in aqueous solution
Poskonin,Badovskaya
, p. 594 - 597 (2003)
The conversion of 5-hydroxy-2(5H)-furanone into succinic acid in aqueous solution has been detected experimentally for the first time, indicating the possibility of forming and hydrolyzing its previously unknown tautomeric forms. The accelerating effect of increased pH values and temperature on the reaction has been established. A scheme is proposed to form succinic acid from 5-hydroxy-2(5H)-furanone.
The Effect of pH on the Reactions of Catalytically Important RhI Complexes in Aqueous Solution: Reaction of [RhCl(tppms)3] and trans-[RhCl(CO)(tppms)2] with Hydrogen (TPPMS = mono-sulfonated triphenylphosphine)
Joo, Ferenc,Kovacs, Jozsef,Benyei, Attila Cs.,Nadasdi, Levente,Laurenczy, Gabor
, p. 193 - 199 (2001)
Hydrolysis and hydrogenation of [RhCl(tppms)3] (1) and trans-[RhCl(CO)(tppms)2] (2) was studied in aqueous solutions in a wide pH range (2 < pH < 11) in the presence of excess TPPMS (3-diphenylphosphinyl-benzenesulfonic acid sodium salt). In acidic solutions hydrogenation of 1 yields a mixture of cis-mer- and cis-fac-[RhClH2(tppms)3] (3a,b) while in strongly basic solutions [RhH(H2O)(tppms)3] (4) is obtained, the midpoint of the equilibrium between these hydride species being at pH 8.2. The paper gives the first successful 1H and 31P NMR spectroscopic characterization of a water soluble rhodium(I)-monohydride (4) bearing only monodentate phosphine ligands. Hydrolysis of 2 is negligible below pH 9 and its hydrogenation results in formation of [Rh(CO)H(tppms)3] (5), which is an analogue to the well known and industrially used hydroformylation catalyst [Rh(CO)H(tppts)3] (6) (TPPTS = 3,3',3"-phosphinetriyltris(benzenesulfonic acid) trisodium salt). It was shown by pH-potentiometric measurements that formation of 5 is strongly pH dependent in the pH5 - 9 range; this gives an explanation for the observed but previously unexplained pH dependence of several hydroformylation reactions. Conversely, the effect of pH on the rate of hydrogenation of maleic and fumaric acid catalyzed by 1 in the 2< pH < 7 range can be adequately described by considering solely the changes in the ionization state of these substrates. All these results warrant the use of buffered (pH-controlled) solutions for aqueous organometallic catalysis.
-
Ince
, p. 155 (1895)
-
Selective C?O Bond Cleavage of Bio-Based Organic Acids over Palladium Promoted MoOx/TiO2
Albarracín-Suazo, Sandra,Nacy, Ayad,Nikolla, Eranda,Pagán-Torres, Yomaira J.,Roberts, Charles A.,Ruiz-Valentín, Génesis,de Lima e Freitas, Lucas Freitas
, p. 1294 - 1298 (2021)
Hydrodeoxygenation chemistries play a key role in the upgrading of biomass-derived feedstocks. Among these, the removal of targeted hydroxyl groups through selective C?O bond cleavage from molecules containing multiple functionalities over heterogeneous catalysts has shown to be a challenge. Herein, we report a highly selective and stable heterogeneous catalyst for hydrodeoxygenation of tartaric acid to succinic acid. The catalyst consists of reduced Mo5+ centers promoted by palladium, which facilitate selective C?O bond cleavage, while leaving intact carboxylic acid end groups. Stable catalytic performance over multiple cycles is demonstrated. This catalytic system opens up opportunities for selective processing of biomass-derived sugar acids with a high degree of chemical functionality.
Selective oxidation of furfural in a bi-phasic system with homogeneous acid catalyst
Li, Xiaodan,Lan, Xiaocheng,Wang, Tiefeng
, p. 97 - 104 (2016)
The selective catalytic oxidation of furfural to 2(5H)-furanone, succinic acid (SA) and maleic acid (MA) was studied. Under optimized conditions, furfural was oxidized to 2(5H)-furanone with a yield of 60–62% in an aqueous/organic bi-phasic system using 1,2-dichloroethane or ethyl acetate as the solvent and formic acid as the catalyst, while the total yield of SA and MA was 15–20%. Compared with other homogeneous and heterogeneous acid catalysts, formic acid gave a much higher selectivity to 2(5H)-furanone because it reacted with hydrogen peroxide to generate performic acid that had a strong oxidizing nature and good solubility in both the aqueous and organic phases. The solvent had a significant influence on the product distribution. A simplified reaction network was established to quantitatively analyze the solvent effect based on the reaction rate constants. In the homogeneous system, the yield of 2(5H)-furanone decreased while the yield of SA increased with an increasing dielectric constant of the solvent. The formic acid/furfural molar ratio, reaction temperature and furfural concentration were optimized for the selective oxidation of furfural to 2(5H)-furanone in the bi-phasic reaction system.
Catalytic oxidation of furan and hydrofuran compounds 8. Synthesis of 5-ethoxycarbonyl-4-hydroxy-3-oxo-2(3H)-furanone by the oxidation of furfural in the system aqueous H2O2-VOSO4-ethanol
Poskonin
, p. 295 - 300 (2008)
The special features of the oxidation of furfural by aqueous hydrogen peroxide in the presence of vanadyl sulfate and ethanol has been studied for the first time. It has been established that this reaction proceeds with the formation of previously unknown
-
Sakurai
, p. 8,10 (1937)
-
Reactions of α-carbanions of lithium acylates with N,N-diethyl-N-chloro- and N,N-diethyl-N-bromoamines
Zorin,Zainashev,Zorin
, (2016)
The interaction of α-carbanions of lithium acylates (prepared via metalation of acetic, butyric, or isobutyric acid with lithium diisopropylamide in tetrahydrofuran under argon atmosphere) with N,N-diethyl-N-chloro- or N,N-diethyl-N-bromoamine has resulte
Effects of hydrophobicity of diffusion layer on the electroreduction of biomass derivatives in polymer electrolyte membrane reactors
Chen, Wei,He, Gaohong,Ge, Feilong,Xiao, Wu,Benziger, Jay,Wu, Xuemei
, p. 288 - 300 (2015)
For the first time, the hydrophobicity design of a diffusion layer based on the volatility of hydrogenation reactants in aqueous solutions is reported. The hydrophobicity of the diffusion layer greatly influences the hydrogenation performance of two model biomass derivatives, namely, butanone and maleic acid, in polymer electrolyte membrane reactors operated at atmospheric pressure. Hydrophobic carbon paper repels aqueous solutions, but highly volatile butanone can permeate in vapor form and achieve a high hydrogenation rate, whereas, for nonvolatile maleic acid, great mass transfer resistance prevents hydrogenation. With a hydrophilic stainless-steel welded mesh diffusion layer, aqueous solutions of both butanone and maleic acid permeate in liquid form. Hydrogenation of maleic acid reaches a similar level as that of butanone. The maximum reaction rate is 340 nmolcm-2 s-1 for both hydrogenation systems and the current efficiency reaches 70%. These results are better than those reported in the literature.
Catalytic oxidation of furan and hydrofuran compounds. 4. Oxidation of furfural by hydrogen peroxide in the presence of sodium molybdate
Grunskaya,Badovskaya,Poskonin,Yakuba
, p. 775 - 780 (1998)
The oxidation of furfural by an aqueous solution of hydrogen peroxide in the presence of sodium molybdate was studied for the first time. Its final products are 2(5H)-furanone and also tartaric, malic, and succinic acids. The process takes place through the formation of peroxide and carbonyl compounds. Kinetic curves for the consumption of the reagents and the accumulation of the reaction products were obtained. In direction oxidation in this system differs substantially from the previously studied reactions of furfural with an aqueous solution of hydrogen peroxide without a catalyst and in the presence of selenium and vanadium compounds. 1999 Kluwer Academic/Plenum Publishers.
Cancer-associated 2-oxoglutarate analogues modify histone methylation by inhibiting histone lysine demethylases
Laukka, Tuomas,Myllykoski, Matti,Looper, Ryan E.,Koivunen, Peppi
, p. 3081 - 3092 (2018)
Histone lysine demethylases (KDMs) are 2-oxoglutarate-dependent dioxygenases (2-OGDDs) that regulate gene expression by altering chromatin structure. Their dysregulation has been associated with many cancers. We set out to study the catalytic and inhibito
-
Fischer,Roedig,Rauch
, (1942)
-
Aqueous-phase catalytic oxidation of furfural with H2O2: High yield of maleic acid by using titanium silicalite-1
Alonso-Fagndez,Agirrezabal-Telleria,Arias,Fierro,Mariscal,Granados, M. Lpez
, p. 54960 - 54972 (2014)
This investigation explores the selective liquid-phase oxidation of furfural to maleic acid (MA) using hydrogen peroxide as an oxidant and titanium silicalite (TS-1) as a catalyst. The effect of temperature and of the concentration of H2O2, furfural and catalyst on the MA yield was studied. The highest yield, 78 mol%, was obtained under the following reaction conditions: 4.6 wt% of furfural, 4.6 wt% of catalyst, a H2O2/furfural mol ratio of 7.5, corresponding to 12.3 wt% of H2O2, 323 K and 24 hours of reaction. To reduce the amount of H2O2 employed, a two-step sequence of reactions was conducted using TS-1 and Amberlyst 70 consecutively as catalysts in the first and second steps, respectively. In this case, a H2O2/furfural mol ratio = 4.4 was required, which is quite close to the stoichiometric ratio (3.0), and a maleic acid yield close to 80% was obtained under 4.6 wt% of furfural, 4.6 wt% of catalyst and 28 h of reaction at 323 K; after 52 h of reaction, the MA yield reached 92%. Fresh and used catalysts were characterised by X-ray diffraction (XRD), Raman spectroscopy, total reflection X-ray fluorescence (TXRF), X-ray photoelectron spectroscopy (XPS), N2 adsorption-desorption isotherms and thermogravimetric analysis. Ti was largely incorporated within the silicalite framework, but the presence of some TiO2 anatase was also confirmed. Ti leaching was observed, especially during the first run but became much less important in successive cycles. Leaching affects both anatase and Ti species within the silicalite framework. Notwithstanding the leaching, when using pure furfural, TS-1 could be reused for six runs without noticeable deactivation, whereas when using furfural directly derived from biomass, weak but visible deactivation occurred upon reutilisation; this deterioration must be related to the presence of some organic products other than furfural.
Levulinic acid upgrade to succinic acid with hydrogen peroxide
Carnevali, Davide,Rigamonti, Marco G.,Tabanelli, Tommaso,Patience, Gregory S.,Cavani, Fabrizio
, p. 98 - 104 (2018)
Levulinic acid is produced from the acidic aqueous degradation of 5-hydroxymethylfurfural, with potential applications in bio-value added chemicals synthesis. Here, we report for the first time, the Baeyer-Villiger oxidation of levulinic acid to succinic acid, with hydrogen peroxide and tungstic acid at mild conditions and without any organic solvent. We investigated the effects of time, amount of reagent-to-catalyst molar ratio and H2O2-to-levulinic acid molar ratio. The maximum succinic acid selectivity was 75% with a levulinic acid conversion as high as 48%, after 6 h at 90 °C. We propose a reaction mechanism based on results obtained from the reactivity of the intermediates. The catalyst interacts with the substrate, forming a cyclic species that enhances the formation of succinic acid versus 3-hydroxypropanoic acid.
-
Ruggli,Maeder
, (1944)
-
-
Schmid,Maschka
, p. 235,238 (1949)
-
Treatment of o-tert-Butyl phenol micro-polluted water with electro-oxidation and microporous aeration: Method development, performance evaluation and mechanism study
Chen
, p. 450 - 454 (2016)
This study investigated the treatment of organic micro-pollutants in drinking water using a combination of electro-oxidation and microporous aeration (EOMA) technique. Results indicated that microporous aeration enhanced the turbulence of reaction solution and improved the efficiency of organic contaminant removal two-fold versus electro-oxidation alone. o-tert-Butyl phenol (OTBP) was used as a representative pollutant. 1600 mL OTBP solution contained 160 mg sodium sulfate and 2 mL 30 % hydrogen peroxide. When the current density was 5 mA cm-2, 1 and 2 mg L-1 o-tert-butyl phenol was removed up to 98.0 and 75.1 %, respectively. The major intermediate products included trimethylacetic acid, succinic acid and other acid. These have much less toxicity than o-tert-butyl phenol. After 30 min, the organics were mineralized completely. Electro-oxidation and microporous aeration was applied to actual source water that was contaminated by complicated organics. No toxicity was shown to algae growth after 15 min of treatment and total organic carbon was removed completely after 30 min.
Tailored design of palladium species grafted on an amino functionalized organozinc coordination polymer as a highly pertinent heterogeneous catalyst
Choudhary,Nishimura,Ebitani
, p. 18687 - 18696 (2014)
The design of a highly active and stable heterogeneous palladium catalyst is gaining a lot of attention because of its increasing importance in the organic syntheses of commodity chemicals. Herein, we report the tailored synthesis of palladium species grafted on a highly stable amino functionalized organozinc coordination polymer (denoted as Pd/AZC) and its extraordinary catalytic performances in the Suzuki-Miyaura coupling (SMC) reaction. It achieved the highest turnover number of 2106 720 (>99% yield) in air among the most reported palladium catalysts for the SMC reaction of bromobenzene. The as-prepared Pd/AZC composite is also successfully applied for the catalysis of Mizoroki-Heck coupling, hydrogenation of nitro, and -CC- functional groups. Since the developed AZC support has thermal stability at least up to 573 K, it possesses high potential for grafting various metal species as catalytically active centers for a wide range of metal-catalyzed reactions. This journal is
HPLC studies on the organic subset of the oscillatory BZ reaction. 2. Two different types of malonyl radicals in the Ce4+-malonic acid reaction
Sirimungkala, Atchara,Foersterling, Horst-Dieter,Noszticzius, Zoltan
, p. 3051 - 3055 (1996)
Applying combined HPLC and NMR techniques, it was found that, besides the already known 1,1,2,2-ethanetetracarboxylic acid (ETA), monomalonyl malonate (MAMA) is also a product of the Ce4+-malonic acid reaction. This is indirect evidence that two different types of organic radicals are formed in the reaction: the alkyl and the carboxylato malonyl radicals. While ETA is a recombination product of two alkyl radicals, MAMA is formed in the recombination of one alkyl and one carboxylato radical.
Anodic Oxidation of Cyclohexanone on Lead Dioxide Electrode in Aqueous Sulfuric Acid Solution
Kunai, Atsutaka,Hatoh, Kazuhito,Hirano, Yoshinobu,Harada, Junji,Sasaki, Kazuo
, p. 1717 - 1722 (1985)
The electrolytic behavior of cyclohexanone was examined by potentiometry with rotating disk electrode as well as by product analyses.The reaction was activation controlled and the reaction orders were first with respect to cyclohexanone and zero for proton.Cyclohexanone reacted by approximately 6 electrons to give adipic acid mainly which accumulated proportionally to the electricity consumed.Changes in temperature, proton concentration, and current denisty resulted in only minor effects on the reaction.In neutral solution, however, the oxidation was suppressed and oxygen evolution dominated.Other electrode materials such as Pt, graphite, RuO2, and PtO2 were inactive.The chemical oxidation with PbO2 itself did not occur.From these facts, reaction mechanism was discussed.
Poly-(styrene sulphonic acid): An acid catalyst from polystyrene waste for reactions of interest in biomass valorization
Alonso-Fagúndez,Laserna,Alba-Rubio,Mengibar,Heras,Mariscal,Granados, M. López
, p. 285 - 294 (2014)
This article reports on the use of poly-(styrene sulphonic acid) (PSSA) prepared by sulphonation of polystryrene waste as catalyst in reactions demanding acid sites. Two different waste derived catalysts (waste to catalyst, WTC) were studied: soluble PSSA (WTC-PSSA) and solid SiO2-PSSA nanocomposite (WTC-SiO2-PSSA). The catalytic properties of these waste derived acid catalysts have been explored in three different reactions of interest in biomass valorization: biodiesel synthesis, xylose dehydration to furfural and furfural oxidation to maleic and succinic acids. The results show that both soluble and nanocomposite WTC catalysts present promising catalytic properties. The WTC-PSSA requires ultrafiltration for reutilization whereas the WTC-SiO2-PSSA can be separated from the reaction mixtures by more usual techniques (centrifugation or conventional filtration). Further research is required for improving the hydrothermal stability of WTC-SiO2-PSSA in order to substantially reduce the leaching of polymer that takes place during the catalytic runs.
SELECTIVE ANODIC OXIDATION OF TETRAHYDROFURAN
Wermeckes, Bernd,Beck, Fritz,Schulz, Harry
, p. 577 - 583 (1987)
The anodic oxidation of tetragydrofuran in acid aqueous electrolytes has been investigated in detail.Selective oxidation to 2-hydroxy tetrahydrofuran has been found to proceed.Under optimum conditions (smooth platinum, high current densities, 200 - 400 mA/cm2, 1 - 6 M THF, 1 M H2SO4, 35 deg C, quasi divided cells), the product was obtained in batch type laboratory scale (60percent of 2 F/mol conversion) with 70percent current efficiency and 95percent selectivity.Only traces of butyrolactone and succinic acid, the dominating products in the case of PbO2 anodes, were found.Oxygen is the main side product.
-
Fischer,Eysenbach
, p. 99,118 (1937)
-
Catalyst for catalytic oxidation of furfural to prepare maleic acid and application thereof
-
Page/Page column 10-12, (2022/02/10)
A catalyst for catalytic oxidation of furfural to prepare maleic acid, relating to the technical field of renewable energy. The catalyst is a mixture of a bromide and a base. A method for preparing the catalyst in catalytic oxidation of furfural to prepare maleic acid. The method includes: mixing the furfural, the bromide-base, an oxidant and a solvent to carry out a reaction to obtain the maleic acid. The present invention has the advantages that the method has a relatively high conversion rate of furfural and a relatively high yield of maleic acid, the conversion rate of furfural is up to 99%, the yield of maleic acid is up to 68.04%; and the catalyst has a high catalytic selectivity and reusability.
Cyanide as a primordial reductant enables a protometabolic reductive glyoxylate pathway
Krishnamurthy, Ramanarayanan,Pulletikurti, Sunil,Yadav, Mahipal,Yerabolu, Jayasudhan R.
, p. 170 - 178 (2022/02/11)
Investigation of prebiotic metabolic pathways is predominantly based on abiotically replicating the reductive citric acid cycle. While attractive from a parsimony point of view, attempts using metal/mineral-mediated reductions have produced complex mixtures with inefficient and uncontrolled reactions. Here we show that cyanide acts as a mild and efficient reducing agent mediating abiotic transformations of tricarboxylic acid intermediates and derivatives. The hydrolysis of the cyanide adducts followed by their decarboxylation enables the reduction of oxaloacetate to malate and of fumarate to succinate, whereas pyruvate and α-ketoglutarate themselves are not reduced. In the presence of glyoxylate, malonate and malononitrile, alternative pathways emerge that bypass the challenging reductive carboxylation steps to produce metabolic intermediates and compounds found in meteorites. These results suggest a simpler prebiotic forerunner of today’s metabolism, involving a reductive glyoxylate pathway without oxaloacetate and α-ketoglutarate—implying that the extant metabolic reductive carboxylation chemistries are an evolutionary invention mediated by complex metalloproteins. [Figure not available: see fulltext.].
Oxidation of cyclohexanone and/or cyclohexanol catalyzed by Dawson-type polyoxometalates using hydrogen peroxide
Dermeche, Leila,Idrissou, Yasmina,Mazari, Tassadit,Moudjahed, Mohammed,Rabia, Cherifa
, (2022/03/07)
The oxidation of cyclohexanone, cyclohexanol or cyclohexanone/cyclohexanol mixture using as catalyst, Dawson-type polyoxometalates (POMs) of formula, α- and β-K6P2W18O62, α-K6P2Mo6W12O62 and α1-K7P2Mo5VW12O62 and hydrogen peroxide, carried out at 90 °C with a reaction time of 20 h, led to a high number of mono- and di-acids which were identified by GC-MS. Levulinic, 6-hydroxyhexanoic, adipic, glutaric and succinic acids, major products were evaluated by HPLC. Regardless of the substrate nature, all POMs exhibited high catalytic activity with 94–99% of conversion, whereas the formation of the different products is sensitively related to both the composition and symmetry of the POMs and the substrate nature. The main products are adipic acid in the presence of α-K6P2Mo6W12O62 and α1-K7P2Mo5VW12O62, levulinic acid in the presence of α1-K7P2Mo5VW12O62 and β-K6P2W18O62 and 6-hydroxyhexanoic acid in the presence of α- and β-K6P2W18O62. Graphical abstract: High catalytic activity was observed with?α- and?β-K6P2W18O62, α-K6P2Mo6W12O62 and α1-K7P2Mo5VW12O62 Dawson-type for the oxidation of cyclohexanone, cyclohexanol or cyclohexanone/cyclohexanol mixture, in the hydrogen peroxide presence, to several oxygenated products. Adipic, levulinic and 6-hydroxyhexanoic acids are the main products. The peroxo- species formed in situ could be the active sites.[Figure not available: see fulltext.]
Labile Photo-Induced Free Radical in α-Ketoglutaric Acid: a Universal Endogenous Polarizing Agent for In Vivo Hyperpolarized 13C Magnetic Resonance
Brindle, Kevin M.,Cheng, Tian,Comment, Arnaud,Gaunt, Adam P.,Hesse, Friederike,Lewis, Jennifer S.,Marco-Rius, Irene
supporting information, (2021/12/09)
Hyperpolarized (HP) 13C magnetic resonance enables non-invasive probing of metabolism in vivo. To date, only 13C-molecules hyperpolarized with persistent trityl radicals have been injected in humans. We show here that the free radical photo-induced in alpha-ketoglutaric acid (α-KG) can be used to hyperpolarize photo-inactive 13C-molecules such as [1-13C]lactate. α-KG is an endogenous molecule with an exceptionally high radical yield under photo-irradiation, up to 50 %, and its breakdown product, succinic acid, is also endogenous. This radical precursor therefore exhibits an excellent safety profile for translation to human studies. The labile nature of the radical means that no filtration is required prior to injection while also offering the opportunity to extend the 13C relaxation time in frozen HP 13C-molecules for storage and transport. The potential for in vivo metabolic studies is demonstrated in the rat liver following the injection of a physiological dose of HP [1-13C]lactate.