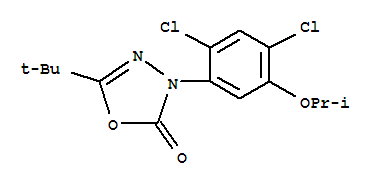
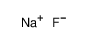Oxadiazon literature
One-pot synthesis of 1,3,4-oxadiazol-2(3H)-ones with CO2 as a C1 synthon promoted by hypoiodite
Yang, Na,Yuan, Gaoqing
, p. 6639 - 6644 (2019/07/16)
A convenient and efficient route has been developed to synthesize 1,3,4-oxadiazol-2(3H)-ones from CO2, hydrazines and aryl or aliphatic aldehydes. Promoted by hypoiodite (IO-) generated in situ from KI and oxidant TBHP, the one-pot synthesis could proceed smoothly to afford the desired products in moderate to high yields. Mechanism studies revealed that nitrile imine was an important intermediate in this transformation. Notably, a commercial herbicide Oxadiazon could be successfully synthesized by this route.
1,3-Dipolar Cycloaddition of Nitrile Imine with Carbon Dioxide: Access to 1,3,4-Oxadiazole-2(3H)-ones
Guo, Chun-Xiao,Zhang, Wen-Zhen,Zhang, Ning,Lu, Xiao-Bing
, p. 7637 - 7642 (2017/07/26)
Efficient synthesis of 1,3,4-oxadiazole-2(3H)-one was achieved by CsF/18-crown-6 mediated 1,3-dipolar cycloaddition of nitrile imine and 2.0 MPa of CO2. CsF/18-crown-6 played a key role in enhancing the reactivity of CO2 as a 1,3-dipolarophile. The practical utility of this transition-metal-free approach to 1,3,4-oxadiazole-2(3H)-one is highlighted by the convenient synthesis of a commercial herbicide Oxadiazon and a MAO B inhibitor.
Method for synthesizing 1,3,4-oxadiazole-2-one compounds of oxadiazon and the like by carbon dioxide
-
Paragraph 0073; 0074; 0075; 0076; 0077; 0078, (2017/07/20)
The invention discloses a method for synthesizing 1,3,4-oxadiazole-2-one compounds of oxadiazon and the like by carbon dioxide. The method comprises: adding an acyl halide hydrazone raw material and a solvent into a high-pressure autoclave; using alkali and an additive as accelerants, introducing carbon dioxide, and performing a stirring reaction for 6 to 24 hours at a temperature of 0 to 70 DEG C; after finishing the reaction, cooling to the room temperature; slowly releasing the unreacted carbon dioxide; after adding water to dilute reaction liquid, carrying out extraction by ethyl acetate; concentrating to obtain a crude product; purifying by column chromatography to obtain the 1,3,4-oxadiazole-2-one compounds. The method disclosed by the invention uses the carbon dioxide for replacing conventional phosgene and carbon monoxide, is safe and simple to operate, low in toxicity, friendly to the environment, simple and easy for obtaining reaction raw materials and reagents, wide in universality of a type of a reaction substrate, simple in post-processing process, high in target product yield and beneficial to industrial production, and is widely applied in synthesis of pesticides, medicines and natural products.
A process for synthesizing oxadiazon
-
Paragraph 0039; 0043-0044, (2017/04/08)
The invention discloses a synthesis technology of oxadiazon. The synthesis technology comprises following steps: (A). carrying out a reaction between 2,4-dichloro-5-isopropoxylbenzhydrazide and methyl chloroformate in an organic solvent to obtain hydrazide methyl ester; (B). enabling the hydrazide methyl ester to be subjected to a cyclization reaction under the effect of a catalyst to obtain the oxadiazon. During the synthesis technology of the oxadiazon, the oxadiazon is synthesized with 2,4-dichloro-5-isopropoxylbenzhydrazide and methyl chloroformate being raw materials, wherein the methyl chloroformate is employed, instead of phosgene or triphosgene, as a carbonylation ring-closing reagent, so that safety potential risk during a conventional phosgene technology is avoided, which enables the synthesis technology of the oxadiazon to be high in safety. In addition, yield of the oxadiazon is high in the invention. An experimental result proves that the yield of the oxadiazon is higher than 97%.