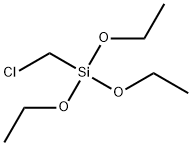
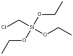
Chloromethyltriethoxysilane literature
Dendrimers with 1, 3, 5-Trisilacyclohexane as Core Unit
Weisheim, Eugen,Neumann, Beate,Stammler, Hans-Georg,Mitzel, Norbert W.
, p. 329 - 334 (2016)
1, 1, 3, 3, 5, 5-Hexavinyl-1, 3, 5-trisilacylohexane [Si(CH=CH2)2CH2]3 was synthesized and hydrosilylated with trichlorosilane to afford the first generation of a dendrimer. Conversion of this molecule with 18 Si-Cl functions on its surface with an excess of vinylmagnesium bromide yielded the 18-fold vinylated dendrimer. The new compounds were identified by elemental analyses, multi-nuclear NMR spectroscopy, and mass spectrometry. Crystal structures were obtained for [Si(CH=CH2)2CH2]3 and [Si(CH2-CH2SiCl3)2CH2]3.
Synthesis and properties of functionalized alkylalkoxysilanes
Lebedev,Minas’yan,Abramkin,Sheludyakov,Kuzmina,Lebedeva,Surikov,Rybakov
, p. 1859 - 1863 (2016)
A method of chloroalkylalkoxysilanes synthesis scalable to pilot production has been proposed. Morpholinotrialkoxysilanes have been prepared and studied as vulcanizing agents for low-molecular silicone rubbers. The reaction of N-morpholinomethyltrialkoxysilanes with triethanolamine has afforded N-[(silatranyl)-methyl]morpholine; it has been studied by X-ray analysis.
Functionalized alpha-amino triethoxy silane and preparation method thereof
-
Paragraph 0058-0062, (2020/12/31)
The invention provides functionalized alpha-amino triethoxy silane and a preparation method thereof. The preparation method comprises the following steps: taking an industrial by-product chloromethyltrichlorosilane as a basic raw material, and reacting with ethanol to generate chloromethyl triethoxysilane; and then carrying out substitution reaction with organic amine containing a double-bond group to prepare the double-bond functionalized alpha-amino triethoxy silane shown in the formula I1. Double-bond functionalized alpha amino triethoxy silane shown as a formula I1 is used as a raw material, and a series of triethoxy silane which contains different functional groups and of which alpha carbon is connected with an amino group and a carbon sulfide bond can be prepared by utilizing a mercapto-alkene click reaction. The industrial byproduct chloromethyl trichlorosilane is fully utilized, and the target product with high purity and high yield can be prepared by using a simple and mild method; the structure of the obtained product contains a plurality of functional groups so that the types of alkoxy silane coupling agents are greatly increased, and the application space is further expanded.
Trialkoxysilanes connected with thioether bond on alpha-carbon and containing different functional groups based on sulfydryl-ene click reaction, and preparation
-
Paragraph 0076-0078, (2019/01/14)
The invention relates to a preparation method of trialkoxysilanes connected with a thioether bond on alpha-carbon and containing different functional groups. The method comprises the steps of reactingchloromethyltrichlorosilane and sodium alkoxide so as to prepare chloromethyltriethoxysilane, then reacting chloromethyltriethoxysilane and thiourea under the action of a catalyst so as to prepare alpha-sulfydrylmethyltriethoxysilane; through performing photo-initiation sulfydryl-ene click reaction, connecting alpha-sulfydrylmethyltriethoxysilane and a double-bond compound, and thus obtaining trialkoxysilanes connected with the thioether bond on the alpha-carbon and containing the different functional groups. According to the method, one of the raw materials is the industrial byproduct chloromethyltrichlorosilane, and the efficient utilization way is opened for chloromethyltrichlorosilane, so that environment protection and energy conversation are realized, the dosage of a toxic catalystis reduced, and the idea of green environment protection is met. According to the preparation method provided by the invention, the raw materials and reaction reagents are easy to get, the product iseasy to separate and purify, and the atom economy is met; the preparation process is simple, the reaction condition is mild, the cost is low, and the preparation method is suitable for large-scale industrial production and application.
Preparation method of alpha-amino triethoxysilane
-
Paragraph 0028; 0052; 0053; 0054; 0055, (2017/08/28)
The invention relates to a preparation method of alpha-amino triethoxysilane. The method comprises the following steps: (1) dissolving sodium ethoxide in a sufficient amount of organic solvent, then uniformly dropwise adding the sodium ethoxide solution into chloromethyl trichlorosilane, allowing the raw materials to react for a period of time at normal temperature after the dropwise addition is completed, carrying out normal pressure distillation to remove ethanol and insoluble substances to obtain chloromethyl triethoxysilane; (2) heating organic amine under the protection of nitrogen until boiling, and then dropwise adding the chloromethyl triethoxysilane prepared in the step (1), allowing the raw materials to react for 1-8 hours at the temperature of 70-200 DEG C after the dropwise addition is completed; filtering the reactant to remove the generated salt after the reaction is ended, distilling at normal pressure to remove the low-boiling-point substance, and then collecting the product at a proper boiling point under reduced pressure distillation, so that the alpha-amino triethoxysilane is obtained. According to the invention, no additional acid absorbent needs to be added, organic solvent is not added, so that the subsequent separation is simple, and the generation of by-products such as polysubstituted compounds of organic amines is less, the utilization rate of the amine is relatively high, and the side reaction of the reaction system is easy to control; the product purity can reach more than 95%, and the yield is over 40%.