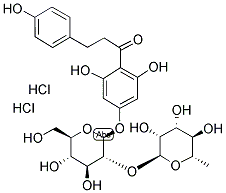
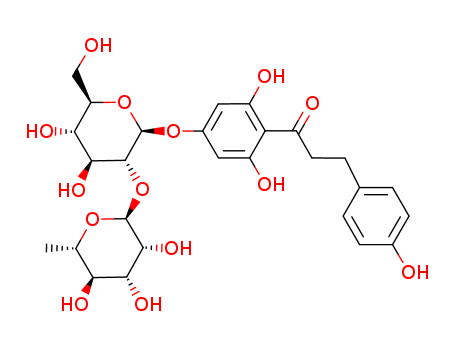
Naringin dihydrochalcone Appearance/Package/Shipping/Storage
package
storage condition
Store in cool & dry place,Keep away from strong light and heat
Naringin dihydrochalcone literature
Synthetic method for naringin dihydrochalcone
-
Paragraph 0022, (2016/10/09)
The invention discloses a synthetic method for naringin dihydrochalcone. The method comprises the following steps that natural extract naringin is adopted as a raw material, alkali dissolution is performed and then filtering is performed; a polarity organic solvent is added, a catalyst is added, hydrogenation reduction is performed on the condition of the certain pressure, the catalyst is filtered away after hydrogen absorption is completed, the pH value is adjusted for crystallization, a product is obtained through filtration, and the naringin dihydrochalcone can be obtained; the liquid phase purity is 98.5% or above, and the molar yield is 96% or above.
Dihydrochalcones: Evaluation as novel radical scavenging antioxidants
Nakamura, Yoshimasa,Watanabe, Shigeo,Miyake, Nobuyuki,Kohno, Hiroyuki,Osawa, Toshihiko
, p. 3309 - 3312 (2007/10/03)
Dihydrochalcones are a family of bicyclic flavonoids, defined by the presence of two benzene rings joined by a saturated three carbon bridge. In the present study, we systematically examined the antioxidant activities of dihydrochalcones against the stable free radical (1,1-diphenyl-2-picrylhydrazyl) and lipid peroxidation in the erythrocyte membrane. All dihydrochalcones exhibited higher antioxidant activities than the corresponding flavanones. The 1H NMR analysis indicated that the active dihydrochalcone has a time-averaged conformation in which the aromatic A ring is orthogonal to the carbonyl group, while the inactive dihydrochalcone such as 2′-O-methyl-phloretin has a strongly hydrogen-bonded phenolic hydroxyl group, suggestive of a coplanar conformation. A hydroxyl group at the 2′-position of the dihydrochalcone A ring, newly formed by reduction of the flavanone C ring, is an essential pharmacophore for its radical scavenging potential.
Naringin dihydrochalcone Upstream and downstream
18916-17-1 Upstream product
18916-17-1 Downstream Products
-
60-82-2
3-(4-Hydroxy-phenyl)-1-(2,4,6-trihydroxy-phenyl)-propan-1-on
-
4192-90-9
4'-(β-D-glucopyranosyloxy)-2',4”,6'-trihydroxydihydrochalcone
-
111316-17-7
4,2′,4′-trihydroxy-6′-methoxydihydrochalcone