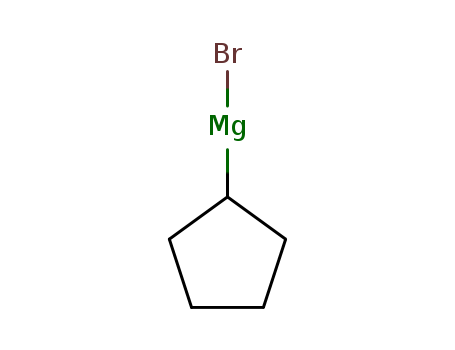Cyclopentylmagnesium Bromide literature
ARYLCYCLOHEXYLAMINE DERIVATIVES AND THEIR USE IN THE TREATMENT OF PSYCHIATRIC DISORDERS
-
Paragraph 0311, (2021/07/02)
Provided herein are arylcyclohexylamine derivatives and their use in the treatment of psychiatric disorders.
Chromium(II)-Catalyzed Diastereoselective and Chemoselective Csp2-Csp3 Cross-Couplings Using Organomagnesium Reagents
Li, Jie,Ren, Qianyi,Cheng, Xinyi,Karaghiosoff, Konstantin,Knochel, Paul
supporting information, p. 18127 - 18135 (2019/11/19)
A simple protocol for performing chromium-catalyzed highly diastereoselective alkylations of arylmagnesium halides with cyclohexyl iodides at ambient temperature has been developed. Furthermore, this ligand-free CrCl2 enables efficient electrophilic alkenylations of primary, secondary, and tetiary alkylmagnesium halides with readily available alkenyl acetates. Moreover, this chemoselective C-C coupling reaction with stereodefined alkenyl acetates proceeds in a stereoretentive fashion. A wide range of functional groups on alkyl iodides and alkenyl acetates are well tolerated, thus furnishing functionalized Csp2-Csp3 coupling products in good yields and high diastereoselectivity. Detailed mechanistic studies suggest that the in situ generated low-valent chromium(I) species might be the active catalyst for these Csp2-Csp3 cross-couplings.
BIARYL PYRAZOLES AS NRF2 REGULATORS
-
Page/Page column 463, (2017/08/01)
The present invention relates to biaryl pyrazole compounds, methods of making them, pharmaceutical compositions containing them and their use as NRF2 regulators.
SYNTHESIS METHOD FOR L-CYCLIC ALKYL AMINO ACID AND PHARMACEUTICAL COMPOSITION HAVING THEREOF
-
Paragraph 0066-0067, (2016/11/17)
A synthesis method for L-cyclic alkyl amino acid and a pharmaceutical composition having the said amino acid are provide in the present disclosure provides. The synthesis method comprises: step A.) preparing a cyclic alkyl keto acid or a cyclic alkyl keto acid salt having Structural Formula (I) or Structural Formula (II), and step B.) mixing the cyclic alkyl keto acid or the cyclic alkyl keto acid salt with ammonium formate, a leucine dehydrogenase, a formate dehydrogenase and a coenzyme NAD+, and carrying out a reductive amination reaction to generate the L-cyclic alkyl amino acid, wherein the Structural Formula (I) is where n1≧1, m1≧0 and the M1 is H or a monovalent cation; the Structural Formula (II) is where n2≧0, m2≧0, the M2 is H or a monovalent cation, an amino acid sequence of the leucine dehydrogenase is SEQ ID No.1.