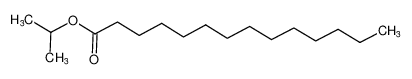
Isopropyl myristate literature
Efficient, stable, and reusable Lewis acid-surfactant-combined catalyst: One-pot Biginelli and solvent-free esterification reactions
Qiu, Yunfeng,Sun, Hongnan,Ma, Zhuo,Xia, Wujiong
, p. 76 - 82 (2014)
Cerium(III) trislaurylsulfonate (Ce(LS)3), a Lewis acid and surfactant combined catalyst, was prepared and characterized by SEM, SEM-EDX, XRD, NMR, FT-IR, TG, and elemental analysis. Ce(LS)3 was found to be stable and efficient to catalyze one-pot Biginelli and solvent-free esterification reactions. Furthermore, Ce(LS)3 is easy to recycle after reaction by pouring into cold water and filtration. Present work will shed deep insight into the understanding of the catalytic nature of LASCs, and extend its application in important organic transformations.
Lipases from Candida antarctica: Unique biocatalysts from a unique origin
Kirk, Ole,Christensen, Morten Wuertz
, p. 446 - 451 (2002)
The specificity of the A-lipase from Candida antarctica (CALA) has been characterized to further clarify the scope of the biocatalyst. The lipase was found to exhibit an almost uniform activity towards various straight-chained primary alcohols and carboxylic acids, only exhibiting a low activity towards very short-chained acids. More interestingly, the enzyme was found to exhibit a high activity towards a surprising diversity of sterically hindered alcohols, including both secondary and tertiary alcohols. These results indicate that CALA can have a unique applicability for the conversion of highly branched substrates where most other lipases fail to display any activity. A new, potentially highly cost-effective, immobilization technology using silica-based granulation has been applied in the immobilization of the B-lipase from the same yeast (CALB). Highly stable particles were obtained with an activity comparable to that of the commercially available immobilized preparations of this enzyme.
Kinetics and mechanism of myristic acid and isopropyl alcohol esterification reaction with homogeneous and heterogeneous catalysts
Yalcinyuva, Tuncer,Deligoez, Hueseyin,Boz, Ismail,Guerkaynak, Mehmet Ali
, p. 136 - 144 (2008)
The reaction of myristic acid (MA) and isopropyl alcohol (IPA) was carried out by using both homogeneous and heterogeneous catalysts. For a homogeneously catalyzed system, the experimental data have been interpreted with a second order, using the power-law kinetic model, and a good agreement between the experimental data and the model has been obtained. In this approach, it was assumed that a protonated carboxylic acid is a possible reaction intermediate. After a mathematical model was proposed, reaction rate constants were computed by the Polymath program. For a heterogeneously catalyzed system, interestingly, no pore diffusion limitation was detected. The influences of initial molar ratios, catalyst loading and type, temperature, and water amount in the feed have been examined, as well as the effects of catalyst size for heterogeneous catalyst systems. Among used catalysts, p-toluene sulfonic acid (p-TSA) gave highest reaction rates. Kinetic parameters such as activation energy and frequency factor were determined from model fitting. Experimental K values were found to be 0.54 and 1.49 at 60°C and 80°C, respectively. Furthermore, activation energy and frequency factor at forward were calculated as 54.2 kJ mol-1 and 1828 L mol-1 s-1, respectively.
ESTERIFICATION OF CARBOXYLIC ACIDS WITH OLEFINS USING A ZEOLITIC MATERIAL HAVING A BEA-TYPE FRAMEWORK STRUCTURE
-
Page/Page column 43; 44; 45, (2019/08/08)
The present invention relates to a catalytic process for the preparation of an ester starting from a carboxylic acid and an alkene using a zeolitic material having a BEA-type framework structure as the catalyst.
Use of Lecitase-Ultra immobilized on styrene-divinylbenzene beads as catalyst of esterification reactions: Effects of ultrasounds
Alves, Joana S.,Garcia-Galan, Cristina,Danelli, Daiane,Paludo, Natália,Barbosa, Oveimar,Rodrigues, Rafael C.,Fernandez-Lafuente, Roberto
, p. 27 - 32 (2015/08/06)
Abstract In this work it was evaluated for the first time, the ester synthesis catalyzed by the phospholipase Lecitase-Ultra immobilized styrene-divinylbenzene beads (MCI-Lecitase), comparing the mechanical stirring and the ultrasonic energy. It was studied the specificity of the enzyme using carboxylic acids from C4 to C18, as well as the effects of alcohol chain, organic solvents, biocatalyst content, reaction temperature and substrate concentration. Caprylic and myristic acids were those with the highest reaction rates and yields, using ethanol as substrate. The shorter the alcohol chain, the higher the enzyme activity. Regarding the secondary alcohols, while MCI-Lecitase had no activity versus isopropanol, using 2-pentanol the activity was similar to that with 1-pentanol. Comparing the agitation systems, MCI-Lecitase presented an initial reaction rate more than 2-times higher in the ultrasound-assisted reaction than under traditional mechanical stirring. Moreover, under ultrasonic energy the maximum rate was achieved using 0.5 M of substrates, while under mechanical stirring the maximum enzyme activity was reached at 0.3 M of substrates. Concerning the operational stability, MCI-Lecitase was quite unstable, losing its activity after 6 reaction cycles. By adding molecular sieves in the reaction medium, MCI-Lecitase retained 30% of its initial activity after 6 cycles.
Composition and method of preparing a tomato-based topical formulation for enhanced healing of burns, ultraviolet and radiation erythema
-
, (2013/03/26)
The present invention is a topical treatment. which can be in the form of cream, ointment, spray or other topically administered composition, which is used to enhance healing of burns, ultraviolet and radiation erythema. The treatment not only reduces pain and inflammation, prevents blistering, and maintains flexibility of the skin, but also accelerates the normal healing process.