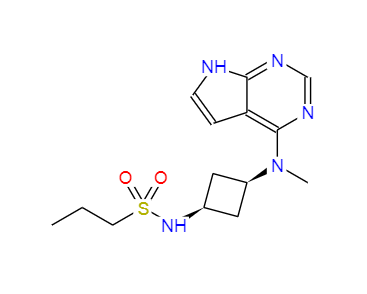
N-{trans-3-[methyl-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]cyclobutyl}propane-1-sulfonamide literature
Identification of N-{cis-3-[Methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]cyclobutyl}propane-1-sulfonamide (PF-04965842): A Selective JAK1 Clinical Candidate for the Treatment of Autoimmune Diseases
Vazquez, Michael L.,Kaila, Neelu,Strohbach, Joseph W.,Trzupek, John D.,Brown, Matthew F.,Flanagan, Mark E.,Mitton-Fry, Mark J.,Johnson, Timothy A.,Tenbrink, Ruth E.,Arnold, Eric P.,Basak, Arindrajit,Heasley, Steven E.,Kwon, Soojin,Langille, Jonathan,Parikh, Mihir D.,Griffin, Sarah H.,Casavant, Jeffrey M.,Duclos, Brian A.,Fenwick, Ashley E.,Harris, Thomas M.,Han, Seungil,Caspers, Nicole,Dowty, Martin E.,Yang, Xin,Banker, Mary Ellen,Hegen, Martin,Symanowicz, Peter T.,Li, Li,Wang, Lu,Lin, Tsung H.,Jussif, Jason,Clark, James D.,Telliez, Jean-Baptiste,Robinson, Ralph P.,Unwalla, Ray
, p. 1130 - 1152 (2018)
Janus kinases (JAKs) are intracellular tyrosine kinases that mediate the signaling of numerous cytokines and growth factors involved in the regulation of immunity, inflammation, and hematopoiesis. As JAK1 pairs with JAK2, JAK3, and TYK2, a JAK1-selective inhibitor would be expected to inhibit many cytokines involved in inflammation and immune function while avoiding inhibition of the JAK2 homodimer regulating erythropoietin and thrombopoietin signaling. Our efforts began with tofacitinib, an oral JAK inhibitor approved for the treatment of rheumatoid arthritis. Through modification of the 3-aminopiperidine linker in tofacitinib, we discovered highly selective JAK1 inhibitors with nanomolar potency in a human whole blood assay. Improvements in JAK1 potency and selectivity were achieved via structural modifications suggested by X-ray crystallographic analysis. After demonstrating efficacy in a rat adjuvant-induced arthritis (rAIA) model, PF-04965842 (25) was nominated as a clinical candidate for the treatment of JAK1-mediated autoimmune diseases.
Development of a Nitrene-Type Rearrangement for the Commercial Route of the JAK1 Inhibitor Abrocitinib
Connor, Christina G.,Deforest, Jacob C.,Dietrich, Phil,Do, Nga M.,Doyle, Kevin M.,Eisenbeis, Shane,Greenberg, Elizabeth,Griffin, Sarah H.,Jones, Brian P.,Jones, Kris N.,Karmilowicz, Michael,Kumar, Rajesh,Lewis, Chad A.,McInturff, Emma L.,McWilliams, J. Christopher,Mehta, Ruchi,Nguyen, Bao D.,Rane, Anil M.,Samas, Brian,Sitter, Barbara J.,Ward, Howard W.,Webster, Mark E.
, p. 608 - 615 (2020/10/09)
The development of a commercial route toward the JAK1 inhibitor abrocitinib is described. The application of a late-stage Lossen rearrangement provided the desired cis-diaminocyclobutane, which was subsequently sulfonylated using a novel water-tolerable triazole sulfonylating reagent to provide the active pharmaceutical ingredient.
METHOD FOR PRODUCING PYRROLO[2,3-D]PYRIMIDINE COMPOUND AND INTERMEDIATE AND METHOD FOR USING THE SAME
-
, (2020/03/10)
PROBLEM TO BE SOLVED: To provide a method for producing a pyrrolo[2,3-d]pyrimidine compound and an intermediate thereof and to provide a method for using the same. SOLUTION: There are provided a method for producing N-((1S,3S)-3-(methyl(7H-pyrrolo[2,3-d)p
TRICYCLIC JANUS KINASE 1 INHIBITORS, AND COMPOSITIONS AND METHODS THEREOF
-
, (2020/05/28)
Provided are novel class of therapeutics that are safe and effective inhibitors of Janus kinase 1 and pharmaceutical composition and methods of preparation and use thereof in the treatment of various diseases and disorders (e. g., inflammatory diseases, immune-mediated diseases or cancer).
PROCESS FOR PREPARATION OF ABROCITINIB
-
Paragraph 0110, (2021/01/23)
The present invention relates to crystalline abrocitinib characterized by X-ray powder diffraction (XRPD) spectrum having peak reflections at about 12.9, 14.7, 19.4, 23.2 and 25.2 ±0.2 degrees 2 theta, and process for its preparation. The present invention relates to amorphous solid dispersion comprising abrocitinib or salt thereof together with at least one pharmaceutically acceptable carrier and process for its preparation.