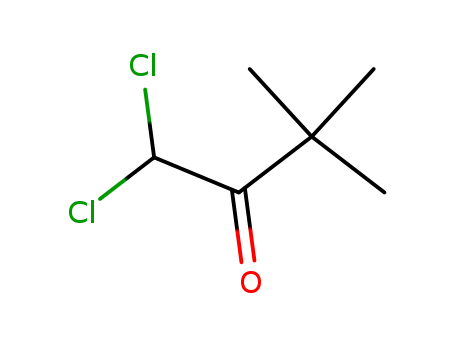
1,1-Dichloropinacolin literature
Method for preparing alpha,alpha-dichloroketone under solvent-free condition
-
Page/Page column 7-8, (2021/06/21)
The invention provides a method for synthesizing an alpha,alpha-dichloroketone compound by taking methyl ketone and sulfonyl chloride as raw materials. The method comprises the following steps: heating a reaction mixture of methyl ketone and sulfonyl chloride to 80 DEG C under a dry air condition, stirring for 4-8 hours, after the reaction is finished, removing sulfonyl chloride from the obtained mixture, and carrying out silica gel column chromatography separation by taking ethyl acetate-hexane as an eluent to obtain the alpha,alpha-dichloroketone compound. The synthesis method provided by the invention has the advantages of extremely high chemical reactivity and selectivity, simple and easily available raw materials, low price, simple operation, no need of any catalyst and solvent, reduction of the synthesis cost and the pollution of organic solvents to the environment, greenness, economy and the like.
Solvent-free preparation of α,α-dichloroketones with sulfuryl chloride
Tu, Dewei,Luo, Juan,Jiang, Wengao,Tang, Qiang
supporting information, (2021/09/15)
An efficient and facile method is reported for the synthesis of a series of α,α-dichloroketones. The direct dichlorination of methyl ketones and 1,3-dicarbonyls using an excess amount of sulfuryl chloride affords the corresponding gem-dichloro compounds in moderate to excellent yields. Moreover, the protocol features high yields, broad substrate scope, and simple reaction conditions without using any catalysts and solvents.
Novel synthesis method of metribuzin intermediate
-
Paragraph 0015; 0019; 0023, (2020/01/25)
The invention discloses a novel synthesis method of a metribuzin intermediate, wherein the intermediate triazinone of metribuzin is produced by using pinacolone (methyl tert-butyl ketone) as an initial raw material through reaction steps of chlorination, hydrolysis, oxidation, condensation and the like. According to the invention, the reaction conditions are mild, and the total yield reaches 92.4%; hydrogen peroxide is used as an oxidizing agent for replacing sodium hypochlorite, and the oxidation reaction is carried out at a room temperature, so that the operation is convenient, and the byproduct is water so as to avoid the discharge of pollutants such as salt-containing wastewater and the like in the production process; and after the reaction is finished, the intermediate and the catalyst are subjected to chromatographic separation so as to recycle the water phase containing the catalyst.
Production process of dichloro pinacolone (by machine translation)
-
Paragraph 0011, (2020/12/29)
The production process is characterized in that the specific production process is as follows: S1: 2 - Chloropentane preparation. S2: pinacolone. S3: pinacolone rectification. S4: synthesis of dichloro-pinacolone. S5: treatment of by-products. In addition, by adopting formaldehyde addition in the chlorination reaction, the reaction rate 3 times or more can be inhibited, and reaction and cooling can be completed between a general reaction requirement 70 - 75 hours by adding formaldehyde, and cooling by adopting chilled water, so that the production efficiency is greatly improved 20 - 25 hours. (by machine translation)