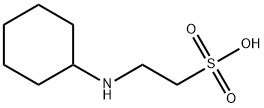
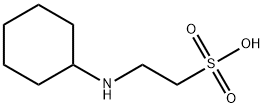
CHES Application
CHES (2- (N-cyclohexylamino) ethanesulfonic acid) is considered a high-quality buffer due to its self buffering properties and biocompatibility. Used to stabilize proteins without interacting with metals. The specific application is as follows:
1. A buffer for determining the activity of mannanase in Antarctic fungal isolates;
2. Buffer solution for DNA enzymatic hydrolysis and labeling of 2 '- deoxyadenosine 3' - monophosphate;
3. As a component of Wilson and Horne extraction buffer, used to characterize rat plasma enzymes.
CHES literature
Preparation method of N-cyclohexyl-2-aminoethanesulfonic acid
-
Paragraph 0015; 0033-0038, (2021/03/13)
The invention discloses a preparation method of N-cyclohexyl-2-aminoethanesulfonic acid, which comprises the following steps: heating cyclohexylamine, 1,2-dichloroethane and sodium sulfite to reflux in the presence of a solvent and a catalyst, reacting at reflux temperature, filtering the reaction solution after the reaction is finished, and acidifying and crystallizing the liquid to obtain the N-cyclohexyl-2-aminoethanesulfonic acid. According to the invention, with the cyclohexylamine, 1,2-dichloroethane and sodium sulfite as raw materials, the product can be prepared just through a one-potone-step process; therefore, the reaction conditions are mild, operation is easy, mastering is easy, products obtained after reaction are easy to treat, and the purity of the products is high and canreach 99% or above.
Method for synthesizing 2 - ring has sulfonic acid process
-
Paragraph 0035-0097, (2019/03/29)
The invention discloses a method for synthesizing 2 - ring has sulfonic acid process, comprising the following steps: the link already amine heating, then drop the heating of 2 - chloroethyl sulfonic acid sodium aqueous solution, stirring and heating to reflux, adding 1 - 3 wt % activated carbon, reflux after filtering is hot, concentrated, adding ethanol, glacial acetic acid to adjust the pH to use 5.0 - 6.0; adding the filtrate 1 - 1.5 times of the volume of ethanol, cooling, filtering; residue to heat of ethanol, reflux, then adding water, filter when it is hot, cooled to 5 - 10 °C, filtering, filter residue with ethanol washing, drying, to obtain 2 - ring has sulfonic acid. The present invention provides synthesis of relatively high process yield, purity is good.
Isolation of nucleic acids
-
, (2008/06/13)
A method for extracting nucleic acids from a biological material such as blood comprises contacting the mixture with a material at a pH such that the material is positively charged and will bind negatively charged nucleic acids and then eluting the nucleic acids at a pH when the said materials possess a neutral or negative charge to release the nucleic acids. The nucleic acids can be removed under mildly alkaline conditions to the maintain integrity of the nucleic acids and to allow retrieval of the nucleic acids in reagents that are immediately compatible with either storage or analytical testing.
Zwitterionic compounds and their n-halo derivatives for use in the treatment of clinical conditions
-
, (2008/06/13)
Zwitterionic compounds selected from: taurine (2-aminoethanesulphonic acid), 2(N-morpholino)ethanesulphonic acid (MES), N-(2-acetamido)iminodiacetic acid (ADA), piperazine-N,N'bis(2-ethanesulphonic acid (PIPES), N-(2-acetamido)-2-aminoethanesulphonic acid (ACES), N,N-bis(2-hydroxyethyl)-2-aminoethanesulphonic acid (BES), 3-(N-morpholino)propanesulphonic (MOPS), N-N[tris(hydroxymethyl)-methyl]-2-aminoethanesulphonic acid (TES), N-2-hydroxyethylpiperazine-N'-2-ethanesulphonic acid (HEPES), N-2-hydroxyethylpiperazine-N'3-propanesulphonic acid (H)EPPS), 2-(cyclohexylamino)ethanesulphonic acid (CHES) or 3-(cyclohexylamino)propanesulphonic acid (CAPS), and their N-halo derivatives can be used separately or in combination in the treatment of related clinical conditions by stimulating myeloperoxidase activity, which in turn stimulates hypochlorous acid production in vivo, which leads inter alia to enhanced leukotriene inactivation.