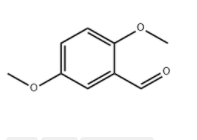
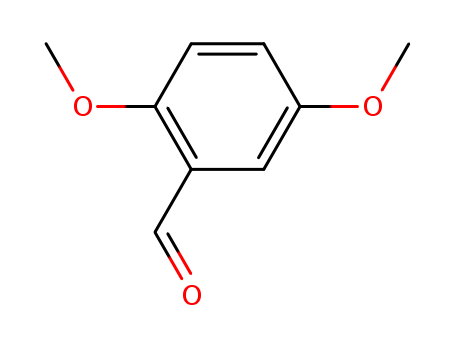
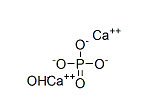
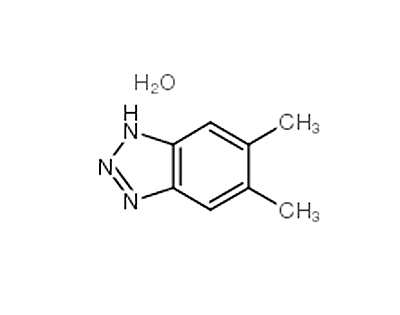
2,5-Dimethoxybenzaldehyde literature
Decomposition of methoxamine in aqueous solution: identification of the decomposition products.
Millard,Priaulx,Shotton
, p. 369 - 373 (1971)
-
Mechanism of Pd(OAc)2/DMSO-catalyzed aerobic alcohol oxidation: Mass-transfer-limitation effects and catalyst decomposition pathways
Steinhoff, Bradley A.,Stahl, Shannon S.
, p. 4348 - 4355 (2006)
Pd(OAc)2 in DMSO is an effective catalyst for the aerobic oxidation of alcohols and numerous other organic substrates. Kinetic studies of the catalytic oxidation of primary and secondary benzylic alcohol substrates provide fundamental insights into the catalytic mechanism. In contrast to the conclusion reached in our earlier study (J. Am. Chem. Soc. 2002, 124, 766-767), we find that Pd(II)-mediated alcohol oxidation is the turnover-limiting step of the catalytic reaction. At elevated catalyst loading, however, the rate of catalytic turnover is limited by the dissolution of oxygen gas into solution. This mass-transfer rate is measured directly by using gas-uptake methods, and it correlates with the maximum rate observed during catalysis. Initial-rate studies were complemented by kinetic analysis of the full-reaction timecourses at different catalyst concentrations. Kinetic fits of these traces reveal the presence of unimolecular and bimolecular catalyst decomposition pathways that compete with productive catalytic turnover.
Preparation method of 2,5-dimethoxyphenylacetic acid
-
Paragraph 0024-0026; 0032-0034; 0040-0042; 0047-0049; 0055, (2021/02/06)
The invention belongs to the technical field of drug synthesis, and particularly relates to a preparation method of 2,5-dimethoxyphenylacetic acid, wherein the method comprises the following steps: A,reacting 1,4-dimethoxybenzene in a formylation system to obtain 2,5-dimethoxybenzaldehyde; B, reacting the 2,5-dimethoxybenzaldehyde obtained in the step A with a reducing agent, extracting a reaction system, then combining organic phases, drying, concentrating under reduced pressure and distilling a crude product to obtain 2,5-dimethoxybenzyl alcohol; C, reacting the 2,5-dimethoxybenzyl alcoholobtained in the step B with a bromination reagent to obtain 2-bromomethyl-1,4-dimethoxybenzene; and D, reacting the 2-bromomethyl-1,4-dimethoxybenzene obtained in the step C with magnesium or butyl lithium and carbon dioxide in a solvent to obtain the 2,5-dimethoxyphenylacetic acid. The yield and the total yield of the 2,5-dimethoxyphenylacetic acid obtained by the method disclosed by the invention are both higher than those of 2,5-dimethoxyphenylacetic acid synthesized by a Willegerdt-Kindler method.
Soluble/MOF-Supported Palladium Single Atoms Catalyze the Ligand-, Additive-, and Solvent-Free Aerobic Oxidation of Benzyl Alcohols to Benzoic Acids
Tiburcio, Estefanía,Greco, Rossella,Mon, Marta,Ballesteros-Soberanas, Jordi,Ferrando-Soria, Jesús,López-Haro, Miguel,Hernández-Garrido, Juan Carlos,Oliver-Meseguer, Judit,Marini, Carlo,Boronat, Mercedes,Armentano, Donatella,Leyva-Pérez, Antonio,Pardo, Emilio
, p. 2581 - 2592 (2021/02/16)
Metal single-atom catalysts (SACs) promise great rewards in terms of metal atom efficiency. However, the requirement of particular conditions and supports for their synthesis, together with the need of solvents and additives for catalytic implementation, often precludes their use under industrially viable conditions. Here, we show that palladium single atoms are spontaneously formed after dissolving tiny amounts of palladium salts in neat benzyl alcohols, to catalyze their direct aerobic oxidation to benzoic acids without ligands, additives, or solvents. With this result in hand, the gram-scale preparation and stabilization of Pd SACs within the functional channels of a novel methyl-cysteine-based metal-organic framework (MOF) was accomplished, to give a robust and crystalline solid catalyst fully characterized with the help of single-crystal X-ray diffraction (SCXRD). These results illustrate the advantages of metal speciation in ligand-free homogeneous organic reactions and the translation into solid catalysts for potential industrial implementation.
Oxidation of Benzylic Alcohols to Aromatic Aldehydes by DMSO/Water/I2: A Chemoselective Oxidation
Adib, Mehdi,Karajabad, Morteza Akherati,Sheikhi, Ehsan
, (2020/03/30)
-
Mechanistic insight into concerted proton-electron transfer of a Ru(IV)-oxo complex: A possible oxidative asynchronicity
Kojima, Takahiko,Kotani, Hiroaki,Shimomura, Hinatsu,Ikeda, Kei,Ishizuka, Tomoya,Shiota, Yoshihito,Yoshizawa, Kazunari
, p. 16982 - 16989 (2020/11/09)
We have thoroughly investigated the oxidation of benzyl alcohol (BA) derivatives by a RuIV(O) complex (RuIV(O)) in the absence or presence of Br?nsted acids in order to elucidate the proton-coupled electron-transfer (PCET) mechanisms in C-H oxidation on the basis of a kinetic analysis. Oxidation of BA derivatives by RuIV(O) without acids proceeded through concerted proton-electron transfer (CPET) with a large kinetic isotope effect (KIE). In contrast, the oxidation of 3,4,5-trimethoxy- BA ((MeO)3-BA) by RuIV(O) was accelerated by the addition of acids, in which the KIE value reached 1.1 with TFA (550 mM), indicating an alteration of the PCET mechanism from CPET to stepwise electron transfer (ET) followed by proton transfer (PT). Although the oxidized products of BA derivatives were confirmed to be the corresponding benzaldehydes in the range of acid concentrations (0-550 mM), a one-electron-reduction potential of RuIV(O) was positively shifted with increases in the concentrations of acids. The elevated reduction potential of RuIV(O) strongly influenced the PCET mechanisms in the oxidation of (MeO)3-BA, changing the mechanism from CPET to ET/PT, as evidenced by the driving-force dependence of logarithms of reaction rate constants in light of the Marcus theory of ET. In addition, dependence of activation parameters on acid concentrations suggested that an oxidative asynchronous CPET, which is not an admixture of the CPET and ET/PT mechanisms, is probably operative in the boundary region (0 mM < [TFA] < 50 mM) involving a one-protoninteracted RuIV(O) H+ as a dominant reactive species.