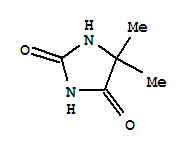
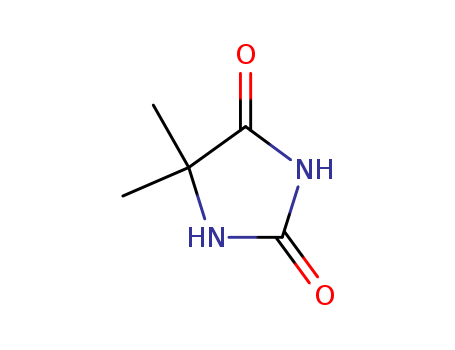
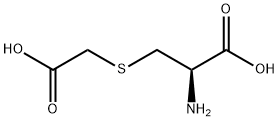
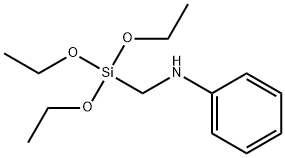
5,5-Dimethylhydantoin literature
-
Bucherer,Brandt
, p. 145 ()
-
Continuous Synthesis of Hydantoins: Intensifying the Bucherer-Bergs Reaction
Monteiro, Julia L.,Pieber, Bartholom?us,Corrêa, Arlene G.,Kappe, C. Oliver
supporting information, p. 83 - 87 (2015/12/26)
A continuous Bucherer-Bergs hydantoin synthesis utilizing intensified conditions is reported. The methodology is characterized by a two-feed flow approach to independently feed the organic substrate and the aqueous reagent solution. The increased interfacial area of the biphasic reaction mixture and the lack of headspace enabled almost quantitative conversions within ca. 30 minutes at 120 °C and 20 bar even for unpolar starting materials. In addition, a selective N(3)-monoalkylation of the resulting heterocycles under batch microwave conditions is reported yielding potential acetylcholinesterase inhibitors.
Synthesis and in vivo hypoglycemic activity of new imidazolidine-2,4-dione derivatives
Hussain, Abid,Kashif, Muhammad Kalim,Naseer, Muhammad Moazzam,Rana, Usman A.,Hameed, Shahid
, p. 7313 - 7326 (2015/09/29)
A series of new 3-arylsulfonylimidazolidine-2,4-diones (2a-2p) were synthesized by reacting imidazolidine-2,4-diones (1a-1e) with arylsulfonyl chlorides in the presence of triethyl amine. The imidazolidine-2,4-diones (1a-1e) were in turn synthesized from the corresponding ketones through Bucherer-Bergs reaction. All the synthesized compounds were characterized on the basis of their spectral (IR, 1H and 13C NMR and MS) and microanalytical data. The hypoglycemic activity of the compounds (2a-2p) was evaluated using alloxanized diabetic rat model. Compound 2a showed an excellent activity with a reduction in the blood glucose level of -286 ± 7 mg/dL after 5 h of drug administration as compared to -270 ± 8 mg/dL for glipizide. The hypoglycemic activity of compound 2b (-268 ± 9 mg/dL) was also comparable to the standard drug. However, only moderate activity was observed for compound 2e.
Mechanochemical preparation of hydantoins from amino esters: Application to the synthesis of the antiepileptic drug phenytoin
Konnert, Laure,Reneaud, Benjamin,De Figueiredo, Renata Marcia,Campagne, Jean-Marc,Lamaty, Frdric,Martinez, Jean,Colacino, Evelina
, p. 10132 - 10142 (2015/02/19)
The eco-friendly preparation of 5- and 5,5-disubstituted hydantoins from various amino ester hydrochlorides and potassium cyanate in a planetary ball-mill is described. The one-pot/two-step protocol consisted in the formation of ureido ester intermediates, followed by a base-catalyzed cyclization to hydantoins. This easy-handling mechanochemical methodology was applied to a large variety of α- and β-amino esters, in smooth conditions, leading to hydantoins in good yields and with no need of purification steps. As an example, the methodology was applied to the "green" synthesis of the antiepileptic drug Phenytoin, with no use of any harmful organic solvent.
Fast halogenation of some N-heterocycles by means of N,N'-dihalo-5,5- dimethylhydantoin
Sandtorv, Alexander H.,Bjorsvik, Hans-Rene
, p. 499 - 507 (2013/05/08)
An instantaneous, selective and high-yielding halogenation process is reported. The method operates with imidazoles, pyrazoles, and indoles under benign reaction conditions. The developed process involves the use of N,N'-dihalo-5,5-dimethylhydantoins (halo=chlorine, bromine, iodine) as halogenation reagents that are activated by catalytic quantities of a strong Bronsted acid. Moreover, the halogenation process is switchable to produce either the mono- or di-halogenated products. Issues related to the reaction mechanism are investigated and a proposal for a reaction mechanism is disclosed.