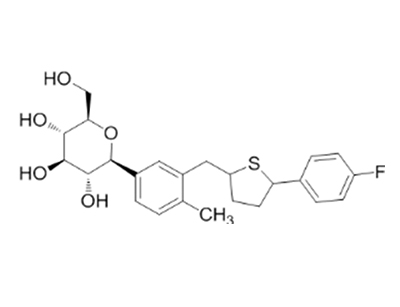
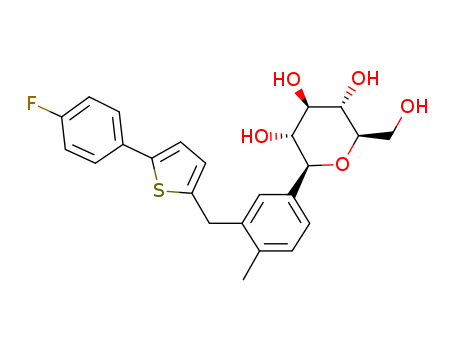
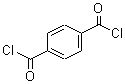
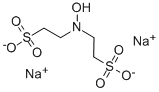
Canagliflozin literature
Preparation method of SGLT-2 inhibitor and intermediate
-
, (2021/05/19)
The invention discloses a preparation method of an SGLT-2 inhibitor and an intermediate. The method comprises the following steps: (1) reacting a compound A with chlorosilane under the action of an acid-binding agent to generate a compound B; (2) mixing the compound B and a compound C to obtain a compound D; and (3) reacting the compound D with a reducing agent and a catalyst to obtain the SGLT-2 inhibitor compound.
Stereoselective Preparation of C-Aryl Glycosides via Visible-Light-Induced Nickel-Catalyzed Reductive Cross-Coupling of Glycosyl Chlorides and Aryl Bromides
Mou, Ze-Dong,Wang, Jia-Xi,Zhang, Xia,Niu, Dawen
supporting information, p. 3025 - 3029 (2021/05/27)
A nickel-catalyzed cross-coupling reaction of glycosyl chlorides with aryl bromides has been developed. The reaction proceeds smoothly under visible-light irradiation and features the use of bench-stable glycosyl chlorides, allowing the highly stereoselective synthesis of C-aryl glycosides. (Figure presented.).
METHOD FOR PRODUCING C-ARYL GLYCOSIDE DERIVATIVE
-
Paragraph 0091-0097, (2021/06/25)
PROBLEM TO BE SOLVED: To provide a method that can produce a C-aryl glycoside derivative stably and inexpensively. SOLUTION: A method for producing a C-aryl glycoside derivative includes the step of mixing, for example, in an organic solvent, the following benzyl-protected C-aryl glycoside derivative (IaA), at least one silyl compound selected from trimethylsilyl chloride and trimethylsilyl bromide, and an alkali metal iodide, thereby producing the following C-aryl glycoside derivative (IIaA). SELECTED DRAWING: None COPYRIGHT: (C)2021,JPOandINPIT
Preparation method of canagliflozin
-
, (2020/02/14)
The invention discloses a preparation method of canagliflozin. According to the method, the reaction of each step in a route is improved, so that the conversion rate of raw materials is increased, andintroduction of potential toxic compounds is avoided. In addition, reaction conditions are mild; operation is simple; purity of an obtained product is high; quality of drugs is improved; and the preparation method is suitable for industrial production.