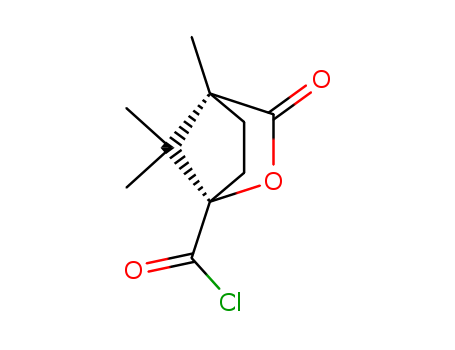
(-)-Camphanic acid chloride literature
188. Bestimmung des Chiralitaetssinns der enantiomeren 2,6-Adamantandiole
Gerlach, Hans
, p. 1815 - 1821 (1985)
The enantiomers of 2,6-adamantanediol (1) are resolved via the diastereoisomeric camphanoates.The (2R,6R)-chirality sense for (-)-1 and (2S,6S) for (+)-1 was determined by chemical correlation with (-)-(1R,5R)-bicyclo<3.3.1>nonan-2,6-dion((1R,5R)-3) of known absolute configuration in the following way: alkylation of the bis(pyrrolidine enamine) of (-)-(1R,5R)-3 with CD2I2 and hydrolysis of the product gives the enantiomer 4 of (4,4-D2)-2,6-adamantanedione.Reduction of 4 with LiAlH4 leads to one enantiomer (Scheme 2) of each of the three diols 5-7 of known absolute configuration.The three diols are themselves configurational isomers due to the presence of the CD2 group, but correspond otherwise entirely to the enantiomeric diols 1.Accordingly, they can also be separated by means of their diastereoisomeric camphanoates to give the diols 5/6 and 7.These samples are easily distinguished and identified by their characteristic 1H-NMR spectra (cf.Fig. 2.).This allows to identify the (2R,6R)- and (2S,6S)-enantiomer of 1 on the basis of their behaviour in the resolution experiment analogous to that of the diols 5/6 and 7, respectively.The diol (-)-1 must have the (2R,6R)-configuration because it forms, like the diols 5/6, with (-)-camphanic acid the diastereoisomeric ester less soluble in benzene.The diol (+)-1 has (2S,6S)-configuration, because it forms, like 7, with (+)-camphanic acid the diastereoisomeric ester less soluble in benzene.The bis(4-methoxybenzoate) of (-)-(2R,6R)-1 shows chiroptical properties which are in accordance with Nakanishi's rule for two chromophores having coupled electric dipol transition moments arranged with a left handed torsion angle.
Chiral bisphosphine ligands based on quinoline oligoamide foldamers: application in asymmetric hydrogenation
Zheng, Lu,Zheng, Dan,Wang, Yanru,Yu, Chengyuan,Zhang, Kun,Jiang, Hua
supporting information, p. 9573 - 9577 (2019/11/20)
A series of chiral bisphosphine ligands were designed and synthesized based on single-handed quinoline oligoamide foldamers. The bisphosphine ligands can coordinate with Rh(cod)2BF4 in a 1 : 1 stoichiometry and the resulted chiral Rh(i) catalysts were applied in the asymmetric hydrogenation of α-dehydroamino acid esters, in which excellent conversions and promising levels of enantioselectivity were achieved.
Irreversible Cysteine-Selective Protein Labeling Employing Modular Electrophilic Tetrafluoroethylation Reagents
Václavík, Ji?í,Zschoche, Reinhard,Klimánková, Iveta,Matou?ek, Václav,Beier, Petr,Hilvert, Donald,Togni, Antonio
supporting information, p. 6490 - 6494 (2017/05/15)
Fluoroalkylation reagents based on hypervalent iodine are widely used to transfer fluoroalkyl moieties to various nucleophiles. However, the transferred groups have so far been limited to simple structural motifs. We herein report a reagent featuring a secondary amine that can be converted to amide, sulfonamide, and tertiary amine derivatives in one step. The resulting reagents bear manifold functional groups, many of which would not be compatible with the original synthetic pathway. Exploiting this structural versatility and the known high reactivity toward thiols, the new-generation reagents were used in bioconjugation with an artificial retro-aldolase, containing an exposed cysteine and a reactive catalytic lysine. Whereas commercial reagents based on maleimide and iodoacetamide labeled both sites, the iodanes exclusively modified the cysteine residue. The study thus demonstrates that modular fluoroalkylation reagents can be used as tools for cysteine-selective bioconjugation.
Asymmetric (4+3) cycloadditions of enantiomerically enriched epoxy enolsilanes
Lo, Brian,Lam, Sarah,Wong, Wing-Tak,Chiu, Pauline
supporting information, p. 12120 - 12123 (2013/01/16)
A f(oxy) allyl: The intermolecular (4+3) cycloaddition of enantiomerically enriched epoxy enolsilanes produces cycloadducts with up to 99 % ee, thus implying the reaction does not proceed by the putative achiral oxyallyl cation intermediate, but through a transiently chiral electrophile which retains the stereochemical information of the epoxide (see scheme; TES=triethylsilyl, Tf=trifluoromethanesulfonyl). Copyright
Synthesis and resolution of 2,2-dimethyl-1,3-diphenyl-1,3-propanediol, a new C2-symmetric and conformationally rigid acyclic diol
Bhowmick,Prasad,Joshi
, p. 851 - 855 (2007/10/03)
Diastereomerically pure (+)- and (-)-2,2-dimethyl-1,3-diphenyl-1,3-propanediols were synthesized starting from diethyl malonate and resolved through diesters of (-)-camphanic acid and also N-carbethoxy-L-proline. The absolute configuration of the (-)-enantiomer was established by X-ray crystallography.