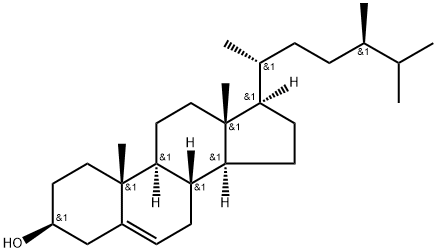
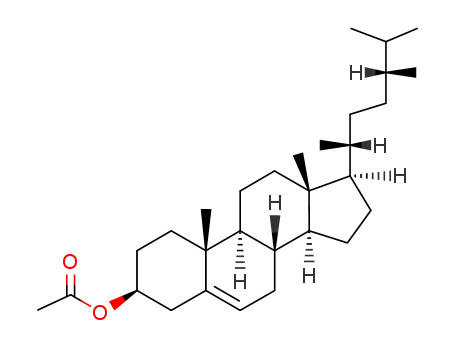
CAMPESTEROL literature
New glycosides of the campesterol derivative from the rhizomes of Tacca chantrieri
Yokosuka, Akihito,Mimaki, Yoshihiro,Sakuma, Chiseko,Sashida, Yutaka
, p. 257 - 265 (2005)
Seven new glycosides of the campesterol derivative (24R,25S)-ergost-5-ene- 3β,26-diol (1-7) were isolated from the rhizomes of Tacca chantrieri (Taccaceae). Their structures were determined by extensive spectroscopic analysis, including 2D NMR data, and a few chemical transformations.
Taccasterosides A-C, novel C28-sterol oligoglucosides from the rhizomes of Tacca chantrieri
Yokosuka, Akihito,Mimaki, Yoshihiro,Sashida, Yutaka
, p. 1396 - 1398 (2004)
Three novel C28-sterol oligoglucosides, named taccasterosides A-C (1-3), were isolated from the rhizomes of Tacca chantrieri (Taccaceae). Their structures were determined by detailed spectroscopic analysis, including 2D NMR data, and a few chemical transformations.
Mechanism of the second methylation in sitosterol side-chain biosynthesis in higher plants: Metabolic fate of 28-hydrogens of 24- methylenecholesterol in Morus alba cell cultures
Okuzumi, Tatsuya,Kaji, Yuko,Hamada, Hiroki,Fujimoto, Yoshinori
, p. 3623 - 3626 (2000)
Biosynthesis of the side-chain of sitosterol in higher plants involves two methylation steps by attack of S-adenosylmethionine. The stereochemical features of the second methylation, namely, of the conversion from 24- methylenecholesterol to isofucosterol in higher plants has been investigated. Feeding studies of synthesized [28E-2H]- and [28Z-2H]-24- methylenecholesterols to cultured cells of Morus alba followed by 2H NMR analysis of the resulting isofucosterol established that the second methylation proceeded in such a manner that addition of the methyl group and proton loss occur on opposite faces of the original Δ(24(28))-double bond. (C) 2000 Elsevier Science Ltd.
Synthetic routes to campesterol and dihydrobrassicasterol: A first reported synthesis of the key phytosterol dihydrobrassicasterol
O'Connell,O'Callaghan,O'Brien,Maguire,McCarthy
experimental part, p. 4995 - 5004 (2012/08/28)
Phytosterols are increasingly used as health supplements in functional foods and are associated with having both positive and negative effects on health. Given this disparity, an investigation of their full individual biological profile is imperative in order to assure food safety. This paper describes the de novo synthesis of pure phytosterols in multigram scale and we report the first synthesis of the key phytosterol dihydrobrassicasterol along with a comparison of routes to campesterol. A detailed spectroscopic analysis is included with full assignment of the 13C NMR spectroscopic data of both compounds, mixtures and their precursors leading to the potential use of NMR spectroscopy as a tool for analysis of these sterol mixtures.
PROCESS FOR RECOVERING STEROLS FROM A CRUDE SOURCE CONTAINING STEROL ESTERS
-
Page/Page column 3, (2008/12/06)
A process of obtaining sterols suitable for human consumption from a crude wood pulping source containing sterol esters is disclosed. The sterols are obtained at high yield and purity. In particular, a process of obtaining sterols at high yield and purity from tall oil pitch (TOP) is disclosed. The sterols obtained can be esterified to sterol esters for use in dietary supplements and as additives for food and beverage products.
Process for recovery of plant sterols from by-product of vegetable oil refining
-
Page/Page column 5-6, (2008/06/13)
The process for recovery of plant sterols and tocopherols from deodorization distillates formed during chemical or physical refining of vegetable oils consists of the following steps: free fatty acids are removed from the deodorization distillate by vacuum distillation or by continuation solvent saponification, after the removal of free fatty acids, the received material is reacted with an aromatic carboxylic acid anhydride at a temperature of 50-150° C., under reduced pressure, after the treatment with anhydride, tocopherols are removed from the mixture, and crystalline free sterols are recovered from the distillation residue containing sterol esters, di- and triglycerides by transesterification.