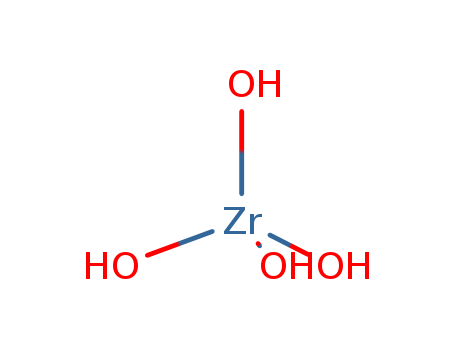
Zirconium hydroxide literature
Influence of the preparation method on the morphological and composition properties of Pd-Au/ZrO2 catalysts and their effect on the direct synthesis of hydrogen peroxide from hydrogen and oxygen
Menegazzo, Federica,Signoretto, Michela,Manzoli, Maela,Boccuzzi, Flora,Cruciani, Giuseppe,Pinna, Francesco,Strukul, Giorgio
, p. 122 - 130 (2009)
Bimetallic Pd-Au samples supported on zirconia were prepared by different methods and tested for the direct synthesis of hydrogen peroxide under very mild conditions (room temperature and atmospheric pressure), outside the explosion range and without halides addition. Further catalytic tests were performed at higher pressure using solvents expanded with CO2. Samples were characterized by N2 physisorption, metal content analysis, XRD, HRTEM combined with X-ray EDS, TPR, and FTIR. The effect of the addition of gold to Pd in enhancing the yield of H2O2 is sensitive to the preparation method: the best catalytic results were obtained by depositing gold by deposition-precipitation (DP) and by introducing in a second step Pd by incipient wetness impregnation. The origin of the differences between samples is discussed. The role of Au in the catalytic reaction seems to be a complex one, changing the chemical composition of the metallic particles, their morphology, and charge of the exposed Pd sites.
On the effect of the strength of acid sites in heterogeneous catalysts on the activity in the skeletal isomerization of n-butane
Shmachkova,Kotsarenko,Paukshtis
, p. 554 - 557 (2004)
The catalytic activity of three groups of acid catalysts different in the nature and strength of acid sites in the skeletal isomerization of n-butane was studied. It was found that the strength of the sites did not correlate with the rate of the reaction.
Synthesis of lead zirconate titanate from an amorphous precursor by mechanical activation
Junmin, Xue,Wang, John,Weiseng, Toh
, p. 139 - 146 (2000)
Many of the chemistry-based processing routes for functional ceramics inevitably involve calcining the chemical-derived precursors at an intermediate/high temperature, in order to form the designed ceramic phase. This is very undesirable, although widely used, as the calcination can result in an extensive degree of crystal growth and particle coarsening at the calcination temperature and therefore ruins almost all the advantages offered by the chemistry-based processing routes, such as an ultrafine particle size and high sintering-reactivity. Using a specifically designed PZT precursor prepared by co-precipitation, it is demonstrated that the precursor-to-ceramic conversion can alternatively be realized by mechanical activation. In this connection, a single phase, nanocrystalline perovskite PZT powder has been successfully derived from an amorphous hydroxide precursor by mechanical activation. The resulting PZT powder was well dispersed, and the particle size was in the range of 30-50 nm, as observed using the scanning electron microscopy and transmission electron microscopy. This is in contrast to the poor particle characteristics, represented by very coarse and irregular particle and agglomerate sizes, for the powder derived from calcination at 750 °C. The activation-triggered PZT powder was sintered to a density of 97.6% theoretical density at 1150 °C for 1 h. Sintered PZT ceramic exhibits a dielectric constant of 927 at room temperature and a peak dielectric constant of approximately 9100 at the Curie point of 380 °C when measured at the frequency of 1 kHz.
Thermoanalytical investigations on hydrous zirconia
Schuster, G.,Braun, G.,Henkel, K.,Querner, G.
, p. 471 - 478 (1988)
The variations in the thermoanalytical curves for three differently produced powders of hydrous zirconia are discussed in connection with X-ray measurements and powder-metallurgical characterization. They were shown to characterize ZrO2-H2O bonding and the thermal treatment for the calcination of hydrous zirconia. They allowed selection of the product with the most favorable microstructure for a high sinter activity, an explanation of the phase formation and phase transformations, and estimations of energy content of the amorphous material and the thermal stability of the tetragonal phase. It was shown that thermal analysis is an appropriate method for the optimizing of ZrO2-powder production.
FT-IR and laser Raman spectroscopic investigation of the formation and stability of low temperature t-ZrO2
Stefanic,Music,Popovic,Sekulic
, p. 391 - 394 (1997)
The influence of the preparation chemistry on the formation and the stability of metastable t-ZrO2 was studied. γ-Irradiation has very small (if any) influence on the t-ZrO2 → m-ZrO2 transition, while increased temperature and pressure caused gradual transition of pure t-ZrO2 to m-ZrO2. Metastable t-ZrO2 containing SO4/2- impurities proved to be more stable, and its transition was not uniform. The mentioned effects were monitored by FT-IR and laser Raman spectroscopy. X-ray diffraction was used as a complementary technique.
Thermodynamic complexity of sulfated zirconia catalysts
Liu, Naiwang,Guo, Xiaofeng,Navrotsky, Alexandra,Shi, Li,Wu, Di
, p. 158 - 163 (2016)
A series of sulfated zirconia (SZ) catalysts were synthesized by immersion of amorphous zirconium hydroxide in sulfuric acid of various concentrations (1–5?N). These samples were fully characterized by X-ray diffraction (XRD), thermogravimetric analysis and mass spectrometry (TGA-MS), and aqueous sulfuric acid immersion and high temperature oxide melt solution calorimetry. We investigated the enthalpies of the complex interactions between sulfur species and the zirconia surface (ΔHSZ) for the sulfated zirconia precursor (SZP), ranging from ?109.46?±?7.33 (1?N) to ?42.50?±?0.89 (4?N) kJ/mol S. ΔHSZ appears to be a roughly exponential function of sulfuric acid concentration. On the other hand, the enthalpy of SZ formation (ΔHf), becomes more exothermic linearly as sulfur surface coverage increases, from ?147.90?±?4.16 (2.14?nm?2) to ?317.03?±?4.20 (2.29?nm?2) kJ/mol S, indicating formation of energetically more stable polysulfate ?species.
Influence of precipitation chemistry and ball-milling on the thermal behavior of zirconium hydroxide
Stefanic,Music,Sekulic
, p. 119 - 133 (1996)
Zirconium hydroxide precipitates, obtained by rapid precipitation at pH 2.5, 7.5, and 10.5, were ball-milled for up to 60 h and then heated inside a differential scanning calorimeter (DSC) at temperatures of up to 600°C. Crystal phases produced after heating were analyzed by FTIR and laser Raman spectroscopy. It was found that without regard to the precipitation pH the first stage of ball-milling caused an increase of the crystallization temperature that resulted in the formation of pure t-ZrO2. The second stage of ball-milling caused a decrease of the crystallization temperature resulting in the formation of m-ZrO2. The ball-milling process also influenced the dependence of the crystallization enthalpy of zirconium hydroxide on the precipitation pH. In the case of zirconium hydroxide precipitated at pH 2.5, the ball-milling caused dehydration and an increase in its hygroscopy. The nature of these effects was discussed. The extension of FTIR spectra to the far infrared region made it possible to distinguish between t-ZrO2 and m-ZrO2 polymorphs by this technique. Also, the influence of laser power on the identification of ZrO2 polymorphs by Raman spectroscopy was elaborated.
CO oxidation by oxygen of the catalyst and by gas-phase oxygen(0.5–15)%CoO/ZrO2
Il’ichev,Fattakhova,Shashkin,Matyshak,Korchak
, p. 300 - 310 (2017)
CO adsorption on (0.5–15)%CoO/ZrО2 catalysts has been investigated by temperature-programmed desorption and IR spectroscopy. At 20°С, carbon monoxide forms carbonyl and monodentate carbonate complexes on Com2+On2- clusters located on the surface of crystallites of tetragonal ZrO2. With an increasing CoO content of the clusters, the amount of these complexes increases and the temperature of carbonate decomposition, accompanied by CO2 desorption, decreases from 400 to 304°С. On the 5%CoO/ZrО2 sample, the carbonyls formed on the Со2+ and Со+ cations and Со0 atoms decompose at 20, 90, and 200–220°С, respectively, releasing CO. At 20°С, they are oxidized by oxygen to monodentate carbonates, which decompose at 180°С. Adsorbed oxygen decreases the temperature of their decomposition on oxidation sites by ~40°C, and the sample remains in an oxidized state ensuring the possibility of subsequent CO adsorption and oxidation. The rate of the oxidation of 5%CoO/ZrО2 containing adsorbed CO by oxygen is higher than the rate of the oxidation of the same sample reduced by carbon monoxide, because the latter reaction is an activated one. In view of the properties of the complexes, it can be concluded that the carbonates decomposing at 180°С are involved in CO oxidation by oxygen from the gas phase in the presence of hydrogen, a process occurring at 50–200°С. The rate-limiting step of this process the decomposition of the carbonates, which is characterized by an activation energy of 77–94 kJ/mol.
Modified zirconia solid acid catalysts for organic synthesis and transformations
Reddy, Benjaram M.,Sreekanth, Pavani M.,Reddy, Vangala R.
, p. 71 - 78 (2005)
The sulfate, molybdate and tungstate promoted ZrO2 catalysts were investigated by X-ray diffraction, NH3-TPD, and Raman spectroscopy and were evaluated for various organic synthesis and transformation reactions. All catalysts exhibit good catalytic activity for synthesis of diphenylureas, coumarines and 1,5-benzodiazepines, acylation of alcohols, phenols and amines, and protection of carbonyl compounds. A series of sulfate, molybdate and tungstate promoted ZrO2 catalysts were prepared by wet impregnation method. To incorporate these promoters to Zr(OH)4, sulfuric acid, ammonium heptamolybdate, and ammonium metatungstate were used as precursors, respectively. Further, a Pt promoted Mo-ZrO2 catalyst was also prepared separately by impregnating with hexachloroplatinic acid. The surface and bulk properties of various promoted ZrO2 catalysts were investigated by means of X-ray powder diffraction, BET surface area, ammonia-TPD, and Raman spectroscopy techniques. The unpromoted ZrO2 when calcined at 873 K exists in the crystalline form with monoclinic phase dominating over the tetragonal phase. Incorporation of various promoters into Zr(OH)4 shows a strong influence on the bulk and the surface properties. Addition of promoters enhanced the tetragonal zirconia phase and the surface acidity. In the case of Pt/Mo-ZrO2 catalyst, a complete tetragonal ZrO2 phase is observed. The ammonia-TPD results indicate that the impregnated sulfate ions show a strong influence on the acidity of ZrO2, which is followed by molybdate. The prepared catalysts were evaluated for various organic synthesis and transformation reactions in the liquid phase. All catalysts exhibit good catalytic activity for synthesis of diphenylureas, coumarines and 1,5-benzodiazepines, acylation of alcohols, phenols and amines, and protection of carbonyl compounds. In particular, the sulfate and molybdate promoted catalysts exhibited excellent catalytic activity.
Evolution of the Catalytic Activity in Pt/Sulfated Zirconia Catalysts: Structure, Composition, and Catalytic Properties of the Catalyst Precursor and the Calcined Catalyst
Manoli, Jean-Marie,Potvin, Claude,Muhler, Martin,Wild, Ute,Resofszki, Gabor,Buchholz, Thomas,Paal, Zoltan
, p. 338 - 351 (1998)
A 3% Pt/sulfated zirconia catalyst was prepared and characterized before and after calcination at 900 K by XRD, XPS, EM, and in the catalytic hydroisomerization of n-hexane. The fresh sample exhibited small but definite catalytic properties. Calcination brought about a dramatic increase of the activity with practically constant high (90-100%) selectivity for hydroisomerization versus cracking. This increased activity was accompanied by the transformation of the predominantly amorphous support to predominantly tetragonal crystals and the wrapping up of most parts of surface Pt atoms into the bulk, as shown by the physical characterization methods. Hence metallic Pt particles exhibited mainly Pt-O rather than Pt-S interactions. S was present as sulfate. Pt-sulfated zirconia was different from traditional bifunctional metal catalysts on acidic supports. We attributed its higher catalytic activity and favorable isomerization selectivity to a few but very active centers, formed by interaction of Pt sites with sulfate groups on the high Miller-index surfaces of ZrO2. Calcination must be essential to create these active sites. H2 dissociating on Pt sites would provide the hydride species that are necessary for isomerization occurring on the acidic (sulfate-zirconia) part of that ensemble. We proposed the name compressed bifunctional sites for these centers of acid-metal cooperation. The assumption of such active sites, the maximum activity as a function of the hydrogen pressure, can also be explained in a consistent way.
Efficient microwave synthesis of novel aromatic esters catalyzed by zirconia and its modified forms: A kinetic study
Thimmaraju,Mohamed Shamshuddin,Pratap,Raja
, p. 99517 - 99528 (2015)
A series of solid acids such as ZrO2, 5%Mo(vi)/ZrO2, 10%Mo(vi)/ZrO2, 20%Mo(vi)/ZrO2 and SO42-/ZrO2 were prepared. These solid acids were characterized by BET, NH3-TPD/n-butylamine back titration, powder-XRD, FT-IR spectroscopy, scanning electron microscopy (SEM), transmission electron microscopy (TEM) and ICP-OES techniques. The catalytic performance of these solid acids was evaluated in the synthesis of novel aromatic esters by the assistance of microwave irradiation and the catalytic activity of these solid acids was compared with pTsOH. The results clearly indicated that the zirconia based solid acids are efficient green catalysts for esterification reactions, which gave a maximum yield of the ester in a shorter reaction time and comparable catalytic activity of the pTsOH Br?nsted acid. Kinetic studies were carried out to calculate the temperature coefficients (1.66 and 1.56) and energy of activation (66.82 kJ mol-1 and 58.93 kJ mol-1) for SO42-/ZrO2 and pTsOH respectively. Pre-adsorption studies revealed that the reaction follows the Langmuir-Hinshelwood mechanism. The SO42-/ZrO2 solid acid catalyst was found to be reactivable and reusable.
Heterogeneous Liquid-Phase Oxidation of Alcohols with Solid Oxidizing Reagents of Vanadium(V) Oxide and Chromium(VI) Oxide Supported on Zirconium(IV) Oxide
Nakamura, Hideo,Matsuhashi, Hiromi
, p. 997 - 1000 (1995)
The oxidizing reagents in a heterogeneous system were obtained by impregnating Zr(OH)4 with NH4VO3 and (NH4)2CrO4 followed by calcination in air at 773 K.These materials (V2O5/ZrO2 and CrO3/ZrO2) converted alcohols into their corresponding aldehydes or ketones at moderate temperatures in a solvent with very high selectivity.Changes in the oxidation states of vanadium and chromium were investigated by a temperature-programmed reduction method (TPR).A linear relation was found between the yield of cyclohexanone formed from cyclohexanol and the amount of reduced vanadium or chromium, determined by TPR.It was confirmed that the active species of V(V) and Cr(VI) were reduced to V(II) and Cr(IV), respectively, in the oxidation process.
Development of a novel mesoporous catalyst UDCaT-6: Kinetics of synthesis of tert-amyl methyl ether (TAME) from tert-amyl alcohol and methanol
Yadav, Ganapati D.,Murkute, Ambareesh D.
, p. 9557 - 9566 (2004)
UDCaT-6, a novel active mesoporous and stable catalyst, was synthesized by generating in situ nanosized acidic centers of chlorosulfonic acid treated zirconia in the pores of highly ordered hexagonal mesoporous silica (HMS). For the first time, we have used chlorosulfonic acid as a source of sulfating agent to treat zirconia in pores of the HMS. The catalyst is characterized by XRD, FTIR, EDAX, SEM, and BET surface area and pore size analysis, and probe reactions. The structural integrity of HMS is maintained in UDCaT-6. The activity and stability of UDCaT-6 was tested in liquid phase alkylation of toluene with benzyl chloride and vapor phase synthesis of tert-amyl methyl ether (TAME) from ferf-amyl alcohol (TAA) and methanol where corrosive acid HCl and water are generated as biproducts. A complete theoretical and experimental analysis is presented and kinetics are evaluated. The model explains the experimental data very well.
Synthesis of solid superacid catalyst with acid strength of H0 ≤ -16.04 1
Hino, Makoto,Arata, Kazushi
, p. 851 - 852 (1980)
A solid superacid catalyst with an acid strength of H0 ≤ -16.04, which was active for reactions of propane and butane, was obtained by exposing Zr(OH)4, prepared by the hydrolyses of ZrOCl2 and ZrO(NO3)2, to 1 N H2SO4 and then calcining in air at 575-650°C.
Synthesis and characterization of mesoporous and nano-crystalline phosphate zirconium oxides
Hernández Enríquez,Cortez Lajas,García Alamilla,Castillo Mares,Sandoval Robles,García Serrano
, p. 425 - 428 (2009)
In this work the preparation and characterization of the materials such as zirconia (ZrO2) and zirconia promoted with phosphate ion (ZrO2-PO43-) is presented. Pure zirconium hydroxide [Zr(OH)4] was synthesized by the sol-gel method using precursors such as zirconium n-butoxide and 1-butanol maintaining a pH 8 during the synthesis. Zr(OH)4 was impregnated with 15 wt.% of the acid agent. Both were calcined in a dynamic air atmosphere for 3 h at 400, 500 and 600 °C. The supports were characterized by thermal analysis, X-ray diffraction, nitrogen physisorption as well as infrared spectroscopy. The results showed a positive effect on the physicochemical properties of the catalytic supports after Zr(OH)4 impregnation with the dopping agent (H3PO4). Phosphate zirconium oxides remained thermically stable after calcination. It was observed that the dopping agent remained firmly attached to the zirconium oxide surface, inhibiting the particle growth and delaying the syntherization of the material and the apparition of the monoclinic phase, obtaining mesoporous and nano-crystalline materials (crystallite size 1.0-6.5 nm) with high surface areas (210-329 m2/g) and tetragonal structure defined for the calcination temperature of 600 °C.
Influence of the preparation and of the activation treatments on the catalytic activity of mechanical mixtures of sulfated zirconia and Pt/Al2O3
Tichit,El Alami,Figueras
, p. 18 - 27 (1996)
Zirconium hydroxides with specific surface areas in the range of 60 to 300 m2/g were obtained by varying between 6 and 12 the final pH of precipitation of the gels and the nature of the precursor salt. These compounds were sulfated by impregnation with 15 and 35 ml of aqueous 0.5 MH2SO4. Their sulfur content was 3 to 5 wt%. Sulfation increases the thermal stabilities of the zirconias by nearly 200 K. The solids remain amorphous up to at least 673 K and then crystallize in the tetragonal and monoclinic phases. The properties of mechanical mixtures of the sulfated zirconias and a Pt/alumina catalyst were evaluated for the isomerization of n-hexane. The study of the conditions of activation show that the highest performances of these catalysts result from a pretreatment under hydrogen at 573 and 623 K when the amounts of H2SO4 added are, respectively, of 35 and 15 ml/g of zirconium hydroxide, whatever their specific surface areas. A close correlation is found between the activities of the catalysts and their specific surface areas. This suggests that the active sites are located at the low coordination sites of zirconia and that their efficiency increases with their dispersion. The acidity determined by IR spectroscopy of NH3 adsorption is essentially of Lewis type after evacuation at 573 K. However, Bronsted acidity results from a reversible redox equilibrium between hydrogen and zirconia. The isomerization of n-hexane is considered to follow a classical bifunctional mechanism.
Improvement of energy storage density with trace amounts of ZrO2 additives fabricated by wet-chemical method
Cui, Chenwei,Pu, Yongping
, p. 495 - 504 (2018)
Lead-free (1-x)[0.6SrTiO3-0.4Na0.5Bi0.5TiO3]-xZrO2 ceramics (STNBT-xZr) were synthesized in single perovskite phase. The average grain size decreased from 3.2 μm (x = 0.1 mol%) to 1.6 μm (x = 0.6 mol%) related to the addition of ZrO2 powders prepared by microwave hydrothermal method. With the increasing of ZrO2 content, the permittivity decreased gradually, and the breakdown strength (Eb) was remarkably improved due to composition induced disturbing of long range ferroelectric order. The increase of Eb led to the improvement of the recoverable energy storage density (Wre) from 1.80 J/cm3 (x = 0.1 mol%) to 2.84 J/cm3 (x = 0.5 mol%). However, when Zr4+ content was more than 0.5 mol%, the Wre decreased from 2.84 J/cm3 to 2.26 J/cm3 (x = 0.6 mol%) due to the reduction of the maximum polarization. The best energy storage properties were achieved in STNBT-xZr ceramic with Zr4+ content of x = 0.5 mol%, which exhibited the Wre of 2.77–2.84 J/cm3 in the range of 4–40 Hz, revealing an excellent frequency stability. All these results demonstrate the STNBT-xZr (x = 0.5 mol%) ceramics are quite promising for frequency-stable energy storage applications.
Metal-reinforced sulfonic-acid-modified zirconia for the removal of trace olefins from aromatics
Kong, Decun,Peng, Qian,Shi, Li,Wang, Xin,Meng, Xuan,Hu, Xiude,Liu, Naiwang
, p. 1644 - 1653 (2020)
Metal-reinforced sulfonic-acid-modified zirconia catalysts were successfully prepared and used to remove trace olefins from aromatics in a fixed-bed reactor. Catalysts were characterized by ICP-OES, N2 adsorption–desorption, X-ray diffraction, thermogravimetric analysis (TGA), and pyridine-FTIR spectroscopy. Different metals and calcination temperatures had great influence on the catalytic activity. Alumina-reinforced sulfated zirconia exhibited outstanding catalytic performance, stable regeneration activity, and giant surface area, and are promising in industrial catalysis. TGA showed that the decomposition of methyl could be attributed to Br?nsted acid sites, and pyridine-FTIR spectroscopy proved the weak Br?nsted sites on these synthesized metal-reinforced sulfated zirconia. Also, a relation between the reaction rate and weak Br?nsted acid density is proposed.
A new solid superacid catalyst prepared by doping ZrO2 with Ce and modifying with sulfate simultaneously
Jong, Rack Sohn,Jun, Seob Lim,Si, Hoon Lee
, p. 1490 - 1491 (2004)
A new solid superacid catalyst, Ce-ZrO2/SO4 2-, having high surface area and thermal stability even after calcination at temperatures of 650-700 °C, is prepared simply by doping ZrO2 with Ce and modifying with sulfate simultaneously. The role of Ce is to form a thermally stable solid solution with zirconia and consequently to give high surface area of the sample.
Hydrogen effect on n-butane isomerization over sulfated zirconia-based catalysts
Sayari, Abdelhamid,Yang, Yong,Song, Xuemin
, p. 346 - 353 (1997)
Iron- and manganese-promoted sulfated zirconia (SFMZ) has been tested as an n-butane isomerization catalyst in the temperature range of 35 to 180°C. The catalytic activity exhibits an induction period whose length is dependent on the reaction conditions. The presence of H2 in the feed stream strongly suppresses n-butane conversion over unpromoted sulfated zirconia (SZ) and over Pt-containing SFMZ (PtSFMZ). However, hydrogen had no effect on n-butane isomerization over SFMZ. These findings were interpreted on the basis of a bimolecular mechanism where unsaturated intermediates (carbenium ions and/or butene) are formed during the break-in period. The role of promoters (Fe and Mn) is not only facilitating the formation of hydrogen-deficient intermediates and their accumulation on the catalyst surface, but also enhancing their stability. The negative effect of hydrogen over PtSFMZ is attributed to the occurrence of atomic hydrogen via the dissociative adsorption of H2 on Pt.
Synthesis and characterization of triflic acid-functionalized mesoporous Zr-TMS catalysts: Heterogenization of CF3SO3H over Zr-TMS and its catalytic activity
Chidambaram,Curulla-Ferre,Singh,Anderson
, p. 442 - 456 (2003)
Triflic acid-functionalized Zr-TMS (zirconium oxide with a mesostructured framework; TMS, transition metal oxide mesoporous molecular sieves) catalysts have been synthesized by functionalizing triflic acid onto the walls of Zr-TMS via post synthesis method. The synthesized materials were characterized by powder XRD, N2-sorption, FT-IR spectroscopy, elemental analysis, solid-state 13C CP and DD/MAS NMR spectroscopy, FT-Raman analysis, NH3-TPD, SEM, TEM, TG-DTA, and DTG techniques. All these results revealed that an ordered Zr-TMS material was synthesized and triflic acid was anchored on the walls of the Zr-TMS. Typical XRD patterns of the Zr-TMS and functionalized Zr-TMS (f-Zr-TMS) showed ordered structures. Synthesized materials showed type IV isotherms. The chemical shift observed (≈119 ppm) and 13C-19F coupling (JC-F≈310 Hz) by 13C DD/MAS NMR showed that the triflic acid was intact on the catalyst framework. According to Raman spectral analysis, triflate was adsorbed on the zirconia surface at all loadings as a tridentate ligand through three equivalent S-O bonds (local C3v symmetry). Ammonia TPD measurements revealed an increase in number of acid sites with an increase in loading of triflic acid. Functionalized amorphous ≡Zr-O-SO2-CF 3 catalysts were also synthesized by an in situ method and SO 42-/ZrO2 was obtained for comparison. The catalytic activity of the materials was tested in the acetalization of ethylacetoacetate and in the benzoylation of biphenyl in a batch reactor at 100 and 150°C, respectively. Recycling was performed in the acetalization of ethylacetoacetate using f-Zr-TMS-30 three times and no major deactivation of the catalyst was observed.
Zirconia supported phosphotungstic acid as an efficient catalyst for resorcinol tert-butylation and n-heptane hydroisomerization
Devassy, Biju M.,Halligudi,Elangovan,Ernst,Hartmann,Lefebvre
, p. 113 - 119 (2004)
The alkylation of resorcinol with tert-butanol was carried out using zirconia supported phosphotungstic acid (PTA) as catalyst in liquid phase conditions. Among the different PTA loaded catalysts, the 15% PTA/ZrO2 calcined at 750 °C was found t
Nanocrystalline yttria-stabilized zirconia fibers from plant fibers and their polymer composites
Das, Rabindra N.,Nad, Sanjukta,Pramanik, Panchanan
, p. 1034 - 1036 (2008)
Nanocrystalline zirconia-8-mol% yttria (yttria-stabilized zirconia (YSZ): ZrO2-8-m% Y2O3) fibers have been prepared from aqueous poly vinyl alcohol (PVA)-zirconium oxy nitrate solution and jute (plant fiber). Soluble Zr and Y ions in PVA solution formed a uniform coating on the surface of jute once it dried completely. Slow hydrolysis of zirconium ion with ammonium hydroxide deposited zirconium hydroxide on the jute surface. Decomposition of the dried zirconium hydroxide-coated jute at high temperature (1200°C/2 h) resulted in the formation of single-phase, nanocrystalline cubic-YSZ with the corresponding average X-ray crystallite size 30-35 nm. Heat-treated fibers have been characterized by X-ray diffraction and scanning electron microscopy. We also prepared polymer composites by incorporating chopped, ground YSZ fibers into epoxy matrix and investigated polymer/fiber interface by transmission electron microscopy analysis.
Effect of ZnO additives and acid treatment on catalytic performance of Pt/WO3/ZrO2 for n-C7 hydroisomerization
Liu, Yan,Guan, Yejun,Li, Can,Lian, Juan,Gan, Geok Joo,Lim, Eng Chew,Kooli, Fethi
, p. 17 - 23 (2006)
The effect of WO3 (5-50 wt%) and ZnO (0.7-22 wt%) on the catalytic properties of Pt/WO3/(ZnO)-ZrO2 for n-heptane (n-C7) hydroisomerization was investigated. The optimized WO3 and ZnO contents are 20 wt% and 3.4 wt%, respectively. The catalytic performance is achieved at 81% n-C7 conversion and 89% C7 isomer selectivity at 250 °C, which is reproducible and can be kept constant over 82 h under reaction conditions. Both WO3 and ZnO can stabilize the tetragonal phase of ZrO2. The Bronsted acid-to-Lewis acid ratio should be optimized to achieve high catalytic performance. The activity for Pt/WO3/ZrO2 using Zr(OH)4 as the catalyst support (n-C7 conversion, 88% at 250 °C) is much higher than that for Pt/WO3/ZrO2 with ZrO2 as the support (n-C7 conversion, 9% at 250 °C) with the same Pt and WO3 loadings. BET, SEM-EDX, and pyridine-FTIR analyses show that acid treatment can successfully enhance the surface area (from 73 to 91 m2/g), increase the number of Bronsted acid sites, and lower the surface Zn:Zr ratio (from 0.43 to 0.15) for ZnO-ZrO2 with 22 wt% ZnO. The yield of C7 isomers is increased from nil to 47% at 300 °C on Pt/WO3/ZnO-ZrO2 catalyst after acid treatment. It is suggested that n-heptane hydroisomerization activity is related to acidity, surface area, and crystalline phase of ZrO2.
Catalytic transfer hydrogenation of furfural to furfuryl alcohol using easy-to-separate core-shell magnetic zirconium hydroxide
Hou, Pan,Ma, Mingwei,Zhang, Peng,Cao, Jingjie,Liu, Hui,Xu, Xingliang,Yue, Huijuan,Tian, Ge,Feng, Shouhua
, p. 2715 - 2722 (2021)
A hollow core-shell magnetic zirconium hydroxide catalyst was synthesized and employed for the catalytic transfer hydrogenation (CTH) ofnumerous biomass-derived platform molecules (furfural and other carbonyl compounds). 93.9% conversion of furfural and 97.3% selectivity of furfuryl alcohol was achieved under mild reaction conditions (160 °C, 4 h, 0.1 g catalyst) with 2-propanol as the H-donor. After 7 times of reaction cycles, the catalyst retained excellent conversion (91.1%) and selectivity (97.8%) and no structural damage was found. Furthermore, a scale-up experiment was carried out, and the results proved that the catalyst has a prospect for industrial applications in the CTH reaction.
Catalytic and surface properties of ZrO2 modified with sulfur compounds
Sohn, Jong Rack,Kim, Hae Won
, p. 361 - 374 (1989)
A series of ZrO2 catalysts modified with sulfur compounds were prepared by treating Zr(OH)4 with sulfate ion and by treating ZrO2 with H2S, CS2, and SO2 followed by oxidation. The redoxidized sulfur species on ZrO2 and the oxidation state of sulfur were investigated by infrared and X-ray photoelectron spectroscopies. It was found that the SO42- species in the highest oxidation state was responsible for enhancing the acidity and catalytic activity of the catalyst. There was a close relationship between the oxidation state of sulfur and the catalytic activity, when 1-butene isomerization was carried out as a test reaction. The surface properties of ZrO2/SO42-, such as specific surface area, phase transition temperture, crystalline structure, acidity and acid strength, were very different from those of ZrO2 not modified with sulfur compounds.
Structural Properties and State of a Zirconium Dioxide Surface Layer Modified with Mе3+ Cations
Kuznetsova,Obukhova,Bondarenko,Fetisova, O. Yu.,Mikhlin, Yu. L.,Kirik,Kuznetsov
, p. 1799 - 1805 (2018)
Abstract: The effect Ме3+ (Al, Y, Sc, Fe, and Mn) cations on the structural properties and state of a zirconium dioxide surface layer is investigated. The thermal stability of the Ме3+ solid solutions based on a metastable zirconium dioxide modification is established via X-ray diffraction analysis. It is shown using X?ray photoelectron spectroscopy that the distribution of surface–volume cationic modifier is determined by the type of cation. In Al-, Fe-, and Mn-ZrO2 systems, modifiers are mainly distributed over a surface; in Y and Sc-ZrО2, modifiers are distributed uniformly. The nature of the cation distribution affects the thermal stability of the solid solutions formed.
Preparation of a novel catalyst UDCaT-5: Enhancement in activity of acid-treated zirconia - Effect of treatment with chlorosulfonic acid vis-a-vis sulfuric acid
Yadav, Ganapati D.,Murkute, Ambareesh D.
, p. 218 - 223 (2004)
UDCaT-5, a zirconia-based catalyst, with high sulfur content (9% w/w) but preservation of the tetragonal phase of zirconia was synthesized for the first time, by using chlorosulfonic acid as a new source for sulfate ions. UDCaT-5 was characterized by using elemental analysis, FTIR, ammonia-TPD, XRD, and BET surface area. Its catalytic activity and stability were evaluated and compared with S-ZrO2 in three different reactions. The characterization and reaction studies show that UDCaT-5 exhibits more superacidity vis-a-vis conventional sulfated zirconia prepared by using sulfuric acid.
Probing the low temperature initiation sites in Fe-, Mn-promoted sulfated zirconia via CO and H2 adsorption
Sayari, Abdelhamid,Yang, Yong
, p. 186 - 190 (1999)
Exposing freshly activated Fe-, Mn-promoted sulfated zirconia to CO at ≤ 50°C induced permanent loss of activity, while adding CO after butane isomerization had a reversible effect, regardless of whether the butane flow was interrupted or not. Similar experiments using dissociated hydrogen instead of CO led to irreversible poisoning in all cases. These findings were analyzed, based on the occurrence of initiation sites that are consumed stoichiometrically and very rapidly on exposure to butane, such initiation sites which are also consumed by CO or dissociated hydrogen, CO which competes effectively for adsorption sites without affecting accumulated reaction intermediates, and such intermediates that are removed in the presence of dissociated hydrogen.
Isomerization of Caryophyllene Oxide Catalyzed by Solid Acids and Bases
Arata, Kazushi,Hayano, Kiyoharu,Shirahama, Haruhisa
, p. 218 - 223 (1993)
The reaction of caryophyllene oxide over eleven catalysts of solid acids and bases gave 4,5-dihydrocaryophyllen-5-one (III), (1R, 5S, 8R, 9R)-4,4,8-trimethyltricyclo<6.3.1.01,5>dodeca-2-en-9-ol (IV), (2S, 5R, 9R)-caryophylla-1(12),8(15)-dien-9-ol (V), (2S, 5R, 9R)-caryophylla-1(12),7-dien-9-ol (VI), and (2S, 5R, 9S)-caryophylla-1(12)-7-dien-9-ol (VII).A large amount of III was formed together with IV over FeSO4 and Zr(SO4)2.Allylic alcohols (V, VI, and VII) were preferentially given by Al2O3-II and TiO2-ZrO2 in >74percent selectivity: 96percent on TiO2-ZrO2.Other catalysts formed three to five species of the products uniformly.
Thiolation of dimethyl sulfide to methanethiol over WO3/ZrO 2 catalysts
Chen, Shiping,Wang, Weiming,Zhang, Yuanhua,Wei, Yucai,Fang, Weiping,Yang, Yiquan
, p. 60 - 65 (2012)
The thiolation of dimethyl sulfide with H2S over a variety of tungsten-zirconia (WO3/ZrO2) catalysts with different contents of WO3 was studied. The maximum yield of methanethiol was obtained at the reaction temperature of 633 K in the presence of 10 wt.%WO 3/ZrO2 catalyst. XRD, BET and TPD characterization results reveal that supporting WO3 species on ZrO2 gives rise to the improvement of both in structure stability and surface acidity. The MT yield increased first and then decreased with the increase of reaction temperature for all of the catalysts due to the decomposition of methanethiol and dimethyl sulfide. The optimum loading of WO3 was found to be 5-10 wt.% (with a surface density of 3.5-4.5 w-atom nm-2). Furthermore, the WO 3/ZrO2 catalyst exhibits high resistance to water.
A discussion of a mechanism for isomerization of n-butane on sulfated zirconia
Garcia,Volpe,Ferreira,Rueda
, p. 263 - 281 (2003)
The mechanism of n-butane isomerization, considering experimental and theoretical works, was studied. MM2 and density functional theory simple calculations were conducted to clarify the nature of the supported active species in sulfated zirconia. A penta-coordinated species, monomeric or even dimeric, would be responsible for forming the most active sites at reaction conditions of n-butane isomerization. No superacidity was present before contact with n-butane. The sites formed at reaction conditions allowed the n-butane dehdyrogenation and its isomerization. At low reaction temperatures, not more than 250°C, additon of hydrogen as carrier gas decreased the conversion comparing it with N2 as carrier gas. At higher temperatures, it increased the activity.
Transesterification of diethyl malonate with benzyl alcohol catalyzed by modified zirconia: Kinetic study
Thimmaraju,Mohamed Shamshuddin,Pratap,Venkatesh
, p. 55 - 65 (2014)
Zirconia and its modified forms such as 10%Mo(VI)/ZrO2, 10%V(V)/ZrO2, 10%W(VI)/ZrO2 and SO4 2-/ZrO2 were prepared and characterized for their physico-chemical properties such as BET for surface area, NH3-TPD and n-butylamine back titration method for total surface acidity, PXRD technique for crystallinity and ICP-OES technique for elemental analysis. These materials were used as catalysts in liquid phase transesterification reaction of diethyl malonate (DEM) with benzyl alcohol (BA). Optimization of reaction conditions such as reaction time, reaction temperature, weight of the catalyst and molar ratio of reactants were carried out to obtain highest possible transester yield. Dibenzyl malonate (DBM) and benzyl ethyl malonate (BEM) were obtained as major products. Highest total transester yield of (88%) was obtained in the presence of 0.75 g of SZ catalyst at a molar ratio of DEM: BA = 1:3, reaction temperature of 393 K and reaction time 5 h. Kinetic studies were carried out to find out the rate of the reaction and energy of activation values for zirconia catalysts, in order to identify a facile catalyst system for this reaction. A possible reaction mechanism was proposed based on the kinetic data and it was observed that Eley-Rideal mechanism fits well for this reaction. Reactivation and reusability studies of the catalysts were also taken up.
Effects of dispersion states of Pb, Ti and Zr ions upon the formation of PbTiO3 and PbZrO3
Takahashi,Kakuta,Ueno
, p. 243 - 254 (1991)
Kinetic studies on the formations of PbTiO3 and PbZrO3 were made using the mixtures of PbO/TiO2 and PbO/ZrO2 with different dispersion states of the constituents. Although the rates of both formations were accel
Blend formed by oxygen deficient MoO3-δ oxides as lithium-insertion compounds
Hashem,Abbas,Abdel-Ghany,Eid,Abdel-Khalek,Indris,Ehrenberg,Mauger,Julien
, p. 744 - 752 (2016)
Oxygen deficient MoxOy 200 nm-thick particles were synthesized by a citrate sol-gel method using ammonium heptamolybdate tetrahydrate as a source of Mo and heat treated with a small fraction of zirconia under reducing atmosphere. The samples were investigated by X-ray powder diffraction (XRD), thermal gravimetric analysis (TGA), scanning electron microscope (SEM) and Raman spectroscopy (RS). The structural analyses show that the composite is a blend formed by layered α-MoO3, orthorhombic oxygen deficient phases MoO3-δ with δ = 0.25 (γ-Mo4O11) and α-ZrMo2O8. The insertion of lithium into the lattice was performed by electrochemical method. Redox peaks were observed for the MoO3-δ composite attributed to distinct Li+ ion insertion/extraction reactions in the MoO3 and Mo4O11 hosts. The cyclic performance revealed improved reversibility, rate capability, and electrochemical stability of the ZrMo2O8-decorated composite with respect to the bare molybdenum oxides. At C/10 rate, the composite delivered a stable reversible capacity of 135 mAh g-1 after the 50th cycle. At a rate of 2C the reversible capacity is maintained at 118 mAh g-1, approximately twice the capacity observed for the MoO3 particles alone.
Silicotungstic acid supported zirconia: An effective catalyst for esterification reaction
Parida,Mallick, Sujata
, p. 77 - 83 (2007)
A series of solid acid catalysts were synthesized by incipient wetness impregnation method by varying the wt% of silicotungstic acid on hydrous zirconia (ZSTA). The prepared catalysts were characterized by PXRD, FTIR, UV-vis DRS, EPMA, BET surface area, acid sites, etc. FTIR and UV-vis DRS studies indicate that the material retain the Keggin-type structure of silicotungstic acid up to 500 °C. The suitability of the materials was studied for acid catalysed esterification reactions using formic, acetic, propionic, n-butyric acid and n-butyl alcohol (NBA), isobutyl alcohol (IBA) and sec-butyl alcohol (SBA). Material with 15 wt% STA on hydrous zirconia having high surface area and acid sites acts as better catalyst for esterification reactions. The esterification of acids with NBA was found to be higher than IBA and SBA. In all the cases, the selectivity for the corresponding esters is nearly100%. The straight-line plot of -ln(1 - conversion) versus reaction time for the reactions carried out at 98 °C supports that the esterification reaction obeys first order kinetics with respect to acid concentration. The reusability study justifies that the catalyst is stable and active.
Properties of nanocrystalline ZrO2-Y2O 3-CeO2-CoO-Al2O3 powders
Dudnik,Tsukrenko,Shevchenko,Ruban,Lopato
, p. 1107 - 1110 (2011)
We have studied the properties of nanocrystalline ZrO2-Y 2O3-CeO2-CoO-Al2O3 powders prepared via hydrothermal treatment of a mixture of coprecipitated hydroxides at 210°C. A number of general trends are identified in the variation of the properties of the synthesized powders during heat treatment at temperatures from 500 to 1200°C. Our results demonstrate that the addition of 0.3 mol % CoO to nanocrystalline ZrO2-based powders containing 1 to 5 mol % Al2O3 allows one to obtain composites with good sinterability at a reduced temperature (1200°C).
Hydrated surface structure and its impacts on the stabilization of t-ZrO2
Wang, Hui,Li, Guangshe,Xue, Yanfeng,Li, Liping
, p. 2790 - 2797 (2007)
We first optimized the preparation conditions to 3.6-6.0 nm ZrO2 in a pure tetragonal structure (t-phase). All samples were characterized by X-ray diffraction, high-resolution transmission electron microscope, thermal analysis, Raman spectra, and infrared spectra. It is found that the surfaces of t-ZrO2 nanostructures were terminated by an amorphous hydration layer co-existing with small amounts of carbonate molecules. With the removal of hydrated surface layers under hydrothermal conditions at T>150 °C, t-ZrO2 nanostructures became thermodynamically unstable, which partially transformed into monoclinic ZrO2 (m-phase). Such a transformation occurs initially at surface regions and then develops into the bulk. High-temperature annealing in air could also remove the hydrated surface layers, which is however followed by a gradual transformation of t-ZrO2 into m-ZrO2 in both bulk and surface regions. These observations are explained in terms of the difference in surface free energies of m-ZrO2 and t-ZrO2 upon H2O adsorption.
Skeletal isomerization of n-pentane over Pt-promoted cesium hydrogen salts of 12-tungstophosphoric acid
Liu, Yanyong,Na, Kyutae,Misono, Makoto
, p. 145 - 153 (1999)
Two kinds of Pt-promoted Cs2.5H0.5PW12O40 (Cs2.5H0.5PW12O40 will be denoted by Cs2.5), Pt directly impregnated on Cs2.5 (Pt/Cs2.5) and a physical mixture of Cs2.5 and Pt/Al2O3 (Pt + Cs2.5), were used to catalyze the isomerization of n- pentane in the presence of hydrogen at 453-573 K. Cs2.5 showed a high initial activity but deactivated rapidly. Addition of Pt greatly suppressed the deactivation and increased the selectivity to isopentane. High stationary conversion (34.8%) and selectivity (96.9%) were obtained by using Pt + Cs2.5 at a relatively low temperature (453 K) and a low hydrogen pressure (0.05 atm, hydrogen/pentane = 1). Under these reaction conditions, the stationary activity and selectivity of Pt + Cs2.5 were significantly higher than those of Pt-promoted H-ZSM-5 or SO4/2-/ZrO2. It was deduced that the remarkable effect of Pt in suppressing the catalyst deactivation was brought about by activated hydrogen, which were formed on Pt, transferred to Cs2.5, and utilized to remove carbonaceous deposits or their precursors. Increase in the hydrogen pressure decreased the initial activity probably due to a decrease in the concentration of pentenes or pentyl carbenium ion.
Synthesis of Solid Superacid of Tungsten Oxide supported on Zirconia and its Catalytic Action for Reactions of Butane and Pentane
Hino, Makoto,Arata, Kazushi
, p. 1259 - 1260 (1988)
A solid superacid catalyst with an acid strength of H0 =/< -14.52 was obtained by impregnating Zr(OH)4 or amorphous ZrO2 with aqueous ammonium metatungstate followed by calcining in air at 800-850 deg C (13 wt.percent W); this catalyst was active for the isomerisations butane to isobutane at 50 deg C, and pentane to isopentane at 30 deg C.
Regioselective side-chain as well as nuclear monobromination of aromatic substrates with N-bromosuccinimide using phosphotungstic acid supported on zirconia as a heterogeneous catalyst
Rajagopal,Siddiqui,Daniel, Thomas,Lahoti,Srinivasan
, p. 165 - 169 (2004)
Regioselective monobromination of aromatic substrates with N-bromosuccinimide has been achieved in excellent isolated yields (84-98%) using phosphotungstic acid supported on zirconia as a novel heterogeneous catalyst. The catalyst has been characterized by means of X-ray diffraction (XRD), scanning electron microscopy (SEM), surface area and acidity measurements. Remarkably, the new catalyst system described brought about the side-chain bromination of aromatics to afford bromomethyl arenes in excellent yields (86-98%) without the need for a radical initiator. Recovery and recylability of the catalyst have been well established.
Estimation of oxyhydroxide specific surface area using the amounts of OH groups adsorbed
Pechenyuk,Matveenko,Semushin
, p. 1582 - 1588 (2001)
A procedure for pH-metric determination of the limiting adsorption of OH groups (AOH) by FeIII, ZrIV, CrIII, and InIII oxyhydroxide hydrogels from 0.1 and 1.0 M solutions of NaCl is described. Data on the molecular area occupied by a single OH group on the hydrogel surface (SOH) and the Sspec values, which were calculated from AOH and SOH. are presented. The Sspec value does not depend on the pH of hydrogel precipitation: the true Sspec value can be determined only from sorption of the OH groups at the actual point of zero charge of the hydrogel. The AOH values for hydrogels were found to change only slightly during aging of hydrogels in electrolyte solutions.
Solubility of Zr(OH)4(am) and the Formation of Zr(IV) Carbonate Complexes in Carbonate Solutions Containing 0.1–5.0?mol·dm?3 NaNO3
Kobayashi, Taishi,Sasaki, Takayuki
, p. 1741 - 1759 (2017)
The solubility of amorphous zirconium hydroxide [Zr(OH)4(am)] was investigated in carbonate solutions containing various concentrations of sodium nitrate. The observed dependences of Zr(IV) solubility on the hydrogen ion concentration (pHc
Methanol Synthesis from CO2 and H2 over CuO-ZnO Catalysts Combined with Metal Oxides under 13 atm Pressure
Xu, Zheng,Qian, Zaihu,Mao, Liqun,Tanabe, Kozo,Hattori, Hideshi
, p. 1658 - 1663 (1991)
The synthesis of methanol from CO2 and H2 in a flow reactor was studied over CuO-ZnO catalysts combined with ZrO2, MgO, Al2O3, or Cr2O3 under a pressure of 13 atm.CuO-ZnO-Al2O3, CuO-ZnO-ZrO2, and CuO-ZnO-MgO showed higher activity and higher selectivity for methanol than did CuO-ZnO.The selectivity for methanol formation became higher as the reaction temperature was lower, or any of the H2/CO2 ratio, the pressure and the space velocity were higher.On the basis of analyses by XRD, XPS and ultraviolet diffused reflectance spectroscopy, the active centers of CuO-ZnO-ZrO2 seem to be a Cu+-ZnO species stabilized by ZrO2.
Zirconia-supported 12-tungstophosphoric acid as a solid catalyst for the synthesis of linear alkyl benzenes
Devassy, Biju M.,Lefebvre,Halligudi
, p. 1 - 10 (2005)
The liquid-phase alkylation of benzene with 1-octene and 1-dodecene was investigated with zirconia-supported 12-tungstophosphoric acid (TPA) as catalysts. We prepared the catalysts, with different TPA loading (5-20 wt% calcined at 750°C) and calcination temperatures (15 wt% calcined from 650 to 850°C), by suspending hydrous zirconia in a methanol solution of TPA, followed by drying and calcination. These catalysts were characterized by X-ray diffraction, DTG-DTA, FTIR pyridine adsorption, NH3-TPD, and 31P MAS NMR spectroscopy measurements. The catalyst with optimum TPA loading (15%) and calcination temperature (750°C) was prepared in different solvents and characterized by 31P MAS NMR spectroscopy. The XRD results indicate that TPA stabilizes the tetragonal phase of zirconia. The catalysts show both Bronsted and Lewis acidity, and 15% TPA on zirconia calcined at 750°C shows the highest acidity. 31P MAS NMR spectra show two types of phosphorous species: one is the Keggin unit and the other is the decomposition product of TPA. The relative amount of each depends on TPA loading, calcination temperature, and the solvent used for the catalyst preparation. Under reaction conditions of 84°C and a benzene/1-olefin molar ratio of 10 (time 1 h), the most active catalyst, 15% TPA, calcined at 750°C, gave more than 98% olefin conversion with selectivity for 2-phenyl octane (53.5%) and 2-phenyl dodecane (47%).
Catalytic dehydration of fructose to 5-hydroxymethylfurfural over a mesoscopically assembled sulfated zirconia nanoparticle catalyst in organic solvent
Wang, Ningning,Yao, Yuan,Li, Wei,Yang, Yan,Song, Zhanxin,Liu, Wentao,Wang, Haijun,Xia, Xiao-Feng,Gao, Haiyan
, p. 57164 - 57172 (2014)
The catalytic dehydration of fructose to 5-hydroxymethylfurfural (HMF) in DMSO was performed over a sequence of mesoscopically assembled sulfated zirconium nanostructures (MASZN) derived from zirconyl chloride with a template as a fastening agent. The materials were characterized by X-ray diffraction, FTIR spectroscopy, NH3 temperature-programmed desorption, pyridine FTIR spectroscopy, field emission scanning electron microscopy, transmission electron microscopy, and N2 sorption. The heterogeneous catalyst MASZN with Lewis-Bronsted acid sites had a superior performance in the dehydration of fructose to HMF. With MASZN-3 as catalyst, a HMF yield of 91.9% with a 98.5% fructose conversion was obtained at 110°C for 120 min in DMSO. Finally, the catalyst MASZN-3 was recycled in four consecutive cycles with scarcely any loss of activity. The excellent catalytic properties together with its easy synthesis, low cost, and nontoxic nature make this MASZN a promising catalyst for the development of new and efficient processes for biomass-based chemicals.
Oxidative esterification of renewable furfural on gold-based catalysts: Which is the best support?
Menegazzo, Federica,Signoretto, Michela,Pinna, Francesco,Manzoli, Maela,Aina, Valentina,Cerrato, Giuseppina,Boccuzzi, Flora
, p. 241 - 247 (2014)
Gold-based catalysts over different supports were investigated in the oxidative esterification of furfural by employing an efficient and sustainable process. The catalytic performances follow the trend: Zirconia-Au > Ceria-Au a‰
Tuning the Lewis acidity of ZrO2for efficient conversion of CH4and CO2into acetic acid
Li, Yufeng,Liu, Bing,Liu, Jie,Wang, Ting,Shen, Yu,Zheng, Ke,Jiang, Feng,Xu, Yuebing,Liu, Xiaohao
, p. 8978 - 8985 (2021/06/02)
The conversion of CH4 and CO2 into acetic acid is a dream reaction, but it remains a great challenge owing to the inertness of both CH4 and CO2. The formation of acetic acid requires efficient activation of CH4 and CO2. In this work, we demonstrated that enhanced acetic acid production from CH4 and CO2 is achieved via improving the Lewis acidity of ZrO2-containing catalysts. Definitely, the best catalyst (SZ-3) exhibits about 14 times higher activity for acetic acid formation than that of pure ZrO2, owing to its strongest Lewis acidity that facilitates the activation of both CH4 and CO2. The mechanism of acetic acid formation is revealed via DFT calculations. CH4 is activated at Lewis acid sites to form Zr-CH3 and O-H species, and subsequently, the O-H species could readily hydrogenate CO3 species formed from CO2 activation at Lewis acid sites to give HCO3, followed by facile coupling with Zr-CH3 yielding acetic acid with a lower energy barrier.
Transfer hydrogenation of furfural to furfuryl alcohol over modified Zr-based catalysts using primary alcohols as H-donors
Wang, Yantao,Zhao, Deyang,Liang, Rui,Triantafyllidis, Konstantinos S.,Yang, Weiran,Len, Christophe
, (2020/12/07)
Catalytic transfer hydrogenation is gaining increasing attention as a promising alternative to conventional hydrogenation with H2. In present work, a series of modified Zr-based catalysts were synthesized and tested for furfural catalytic transfer hydrogenation into furfuryl alcohol (FA). The results indicated that more than 13 % of furfural conversion and furfuryl alcohol yield could be achieved with modified zirconium hydroxide (mZrH) at 140 °C when compared with zirconium hydroxide (ZrH) using ethanol as H-donor and solvent in continuous flow regime, and the activity could be further enhanced by increasing the reaction temperature or Ru loading on the catalyst. The best result of 92 % furfural conversion with ~99 % FA selectivity was obtained at 150 °C with 6% Ru/mZrH as catalyst, and the productivity of FA is 5.5 mmol g?1 h?1 which is 2 times higher than that reported with ZrH in batch. Moreover, long-term stability study of the catalysts indicated that 6% Ru/mZrH not only performs a better activity, but also a better stability than 6% Ru/ZrH. Characterizations of the catalysts by BET, XRD, EA, IR, SEM-EDS, XPS and CO2 adsorption indicated that zirconium hydroxide (ZrH) was successfully modified with hydroxylamine, leading to significantly change of its morphology and basic sites. And the deactivation of the catalysts was due to both the leaching of Ru and the deposition of side-products on its surface.
Fluorite-like hydrolyzed hexanuclear coordination clusters of Zr(IV) and Hf(IV) with syn-syn bridging N,N,N-trimethylglycine in soft crystal structures exhibiting cold-crystallization
Matsuoka, Moe,Takao, Koichiro,Tsushima, Satoru
, (2021/09/27)
Tetravalent metal ions are hydrolyzed under presence of N,N,N-trimethylglycine hydrochloride (betaine hydrochloride, [Hbet]Cl) in aqueous solutions to afford [M6(μ3-O)4(μ3-OH)4(μ-bet)8(κ-bet)4(H2O)4]12+ (M4+ = Zr4+ (1), Hf4+ (2)) as hydrated perchlorate salts. These compounds were characterized by single crystal X-ray diffraction, elemental analysis and IR spectroscopy. As a result, we have found that fluorite-like [M6O8] coordination clusters are formed through octahedral arrangement of six M4+ linked by μ3-O atoms. Additionally, each pair of neighboring M4+ are connected by the μ-bet ligand through a syn-syn bridging coordination of its carboxylate moiety. This interaction seems to prevent further growth of the fluorite structure leading to formation of MO2. It was difficult to directly distinguish each μ3-O atom to be μ3-OH? or μ3-O2? due to its strongly anisotropic thermal displacement in the obtained structures. Bond valence sum analysis suggested that four μ3-OH? and four μ3-O2? are alternately arranged in the [M6O8] core motifs. Indeed, such a symmetric structure of [M6(μ3-O)4(μ3-OH)4] was confirmed in another phase of 1 at 296 K, where 1 transforms to a monoclinic structure (1′). The number of ClO4? counteranions found in the structure determination is not enough to compensate + 12 charge of [M6(μ3-O)4(μ3-OH)4(μ-bet)8(κ-bet)4(H2O)4]12+ in any unit cells of 1, 1′ and 2. Instead, large solvent/ion accessible voids have been actually observed in their crystal structures, indicating that the missing ClO4? are located therein and are significantly disordered to make them invisible in the crystallography. DSC analysis revealed that ClO4? and H2O/H3O+ in the crystal lattice of 1 undergo unique structure relaxation and rearrangements pronounced by cold-crystallization to induce the phase transition from 1 to 1′ with elevating temperature.