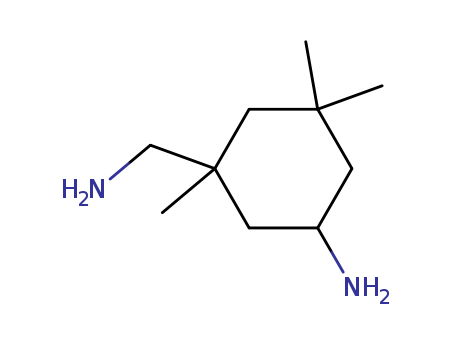
Isophorondiamine literature
Method for preparing IPDI (isophorone diisocyanate)
-
, (2019/06/13)
The invention discloses a method for preparing IPDI (isophorone diisocyanate). The method comprises the following steps: (1) isophorone and hydrogen cyanide are subjected to a reaction in the presenceof a catalyst to obtain isophorone nitrile; (2) isophorone nitrile obtained in step (1), ammonia and hydrogen are subjected to a reaction in the presence of a catalyst to obtain isophorone diamine; (3) isophorone diamine is subjected to a phosgenation reaction to obtain IPDI, wherein the content of impurities containing secondary amine groups in isophorone diamine subjected to the phosgenation reaction in step (3) is smaller than or equal to 0.5wt%, preferably smaller than or equal to 0.3wt%, and more preferably smaller than or equal to 0.1wt%. By means of the method, content of hydrolyzed chlorine in the IPDI product can be effectively reduced, the yellowing resistance of the product is effectively improved, and the harm that downstream products are unqualified due to existence of hydrolyzed chlorine in the product is reduced.
Synthesis method of isophorone diamine
-
Paragraph 0040; 0041; 0043; 0044, (2018/03/24)
The invention relates to a synthesis method of isophorone diamine. The method includes the following steps that 1, in the presence of a catalyst, isophorone (IP) and nitromethane (NM) are subjected to addition reaction, and a nitromethyl-isophorone (NMIP) crude product is prepared; 2, the nitromethyl-isophorone (NMIP) crude product prepared in the step 1 is purified, and a nitromethyl-isophorone(NMIP) pure product is prepared; 3, the nitromethyl-isophorone (NMIP) pure product prepared in the step 2 is dissolved in a solvent, organic acid ammonia salt is added, reaction is conducted in the presence of a catalyst to generate isophorone diamine synthetic liquid, and finally, an isophorone diamine product is obtained through purification. According to the method, highly toxic hydrocyanic acid does not serve as necessary raw materials, high-pressure hydrogen and liquid ammonia are not used as raw materials for reaction, the technological process is safe and environmentally friendly, investments are saved, and the economic benefits are remarkable.
Process For Preparing Amino Compounds From Nitrile Compounds
-
Paragraph 0074; 0075; 076, (2018/12/11)
The present invention relates to a process for hydrogenating nitrile compounds to amino compounds, in which the cross-sectional loading of the reactor during the hydrogenation is less than or equal to 4.0 kg/m2*s, based on the liquid phase.
A process for preparing isophorone diamine
-
Paragraph 0049-0052; 0054, (2017/08/26)
The invention discloses a method for preparing isophorone diamine, and is characterized in that the method comprises the following steps: 1) under the condition of an organic amine catalyst, with isophorone and nitromethane as raw materials, synthesizing 3-nitromethyl-3,5,5-trimethylcyclohexanone, wherein the reaction temperature is 0 DEG C-150 DEG C, and the amount of the organic amine catalyst is 1-300% of the mass of the substance amount of isophorone; and 2) under conditions of liquid ammonia, hydrogen and a metal cobalt catalyst, allowing 3-nitromethyl-3,5,5-trimethylcyclohexanone to undergo a reaction to generate isophorone diamine, wherein the reaction temperature is 30-300 DEG C, the liquid ammonia amount is 1-50 times of the substance amount of 3-nitromethyl-3,5,5-trimethylcyclohexanone, and the hydrogen pressure is 0.1-10 MPa. The nitromethane with lower toxicity is used for replacing highly-toxic hydrogen cyanide or thiocyanate, and thus the method is a technical route which is safer in process and friendlier to the environment.