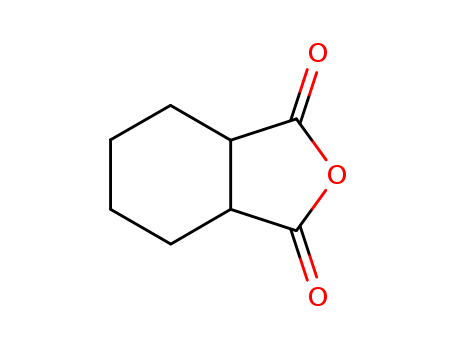
Hexahydrophthalic anhydride literature
Synthesis method of trans 1 and 2 - cyclohexane dicarboxylic acid monomethylester
-
Paragraph 0033-0039; 0040-0043, (2021/08/25)
The invention provides a synthesis method of trans 1 and 2 - cyclohexane dicarboxylic acid monomethylester, and belongs to the field of organic synthesis. The trans 1-2 -cyclohexane dicarboxylic acid monomethylester synthesis method comprises the following steps: trans-cyclohexane -1 and 2 -dicarboxylic acid anhydride. The methanol and solvent is added to the reaction vessel, stirred and purified to obtain the target product trans 1, 2 - cyclohexanedicarboxylate, wherein the solvent is any one or more of tetrahydrofuran, 2 - methyltetrahydrofuran, benzene, toluene, methylene chloride, chloroform, ethyl acetate, methyl acetate or diethyl ether. Since a specific solvent is selected for the reaction solvent, racemization does not occur in the process of synthesizing trans 1 and 2 - cyclohexane dicarboxylic acid monomethyl ester, and the trans ee and 1 cyclohexane dicarboxylic acid monomethyl ester with higher 2 - value can be finally obtained.
RECYCLABLE POLYMERS BASED ON RING-FUSED GAMMA-BUTYROLACTONES
-
Page/Page column 34; 39, (2020/02/23)
The invention discloses a class of new polymers, trans-ring-fused poly(4-hydroxybutyrate)s (RF-P4HB) that exhibit a unique set of properties, including robust thermal stability and mechanical strength, quantitative recyclability to the building block monomers via thermolysis and/or chemical catalysis, and convenient production from the chemical ring-opening polymerization under ambient temperature and pressure. Another unique property is the formation of crystalline stereocomplexed polymers with high melting temperature upon mixing the two enantiomeric RF-P4HB chains via stereocomplexing co-crystallization. This invention also provides the corresponding ring-fused lactone monomer structures that enable the synthesis of the RF-P4HB polymers, through trans-fusing of rings to the parent γ-butyrolactone ring. Furthermore, a polymerization or copolymerization process for the synthesis of RF-P4HB polymers and copolymers is disclosed.
Catalytic hydrogenation products of aromatic and aliphatic dicarboxylic acids
Shinde, Sunil B.,Deshpande, Raj M.
, p. 1137 - 1142 (2019/04/05)
Hydrogenation of aromatic dicarboxylic acids gave 100 % selectivity to respective cyclohexane dicarboxylic acid with 5 % Pd/C catalyst. 5 % Ru/C catalyst was observed to give over hydrogenation products at 493 K and at lower temperature (453 K) the selectivity for cyclohexane dicarboxylic acids was increased. Hydrogenation of phthalic acid with Ru-Sn/Al2O3 catalyst was observed to give phthalide instead of 1,2-benzene dimethanol or 2-hydroxy methyl benzoic acid. Ru-Sn/Al2O3 catalyst selectively hydrogenated the carboxylic group of cyclohexane dicarboxylic acids to give cyclohexane dimethanol. Use of proper catalysts and reaction conditions resulted in desired products.
Exploring the active conformation of cyclohexane carboxylate positive allosteric modulators of the type 4 metabotropic glutamate receptor
Rovira, Xavier,Harrak, Youssef,Trapero, Ana,Gonzlez-Bulnes, Patricia,Malhaire, Fanny,Pin, Jean-Philippe,Goudet, Cyril,Giraldo, Jesffls,Llebaria, Amadeu
, p. 2685 - 2698 (2015/02/02)
The active conformation of a family of metabotropic glutamate receptor subtype 4 (mGlu4) positive allosteric modulators (PAMs) with the cyclohexane 1,2-dicarboxylic scaffold present in cis-2-(3,5-dichlorophenylcarbamoyl)cyclohexanecarboxylic acid (VU0155041) was investigated by testing structurally similar six-membered ring compounds that have a locked conformation. The norbornane and cyclohexane molecules designed as mGlu4 conformational probes and the enantiomers of the trans diastereomer were computationally characterized and tested in mGlu4 pharmacological assays. The results support a VU0155041 active conformation, with the chair cyclohexane having the aromatic amide substituent in an axial position and the carboxylate in an equatorial position. Moreover, the receptor displays enantiomeric discrimination of the chiral PAMs. The constructed pharmacophore characterized a highly constrained mGlu4 allosteric binding site, thus providing a step forward in structure-based drug design for mGlu4 PAMs.