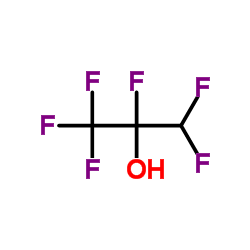
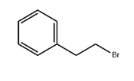
1,1,1,3,3,3-Hexafluoro-2-propanol literature
1,1,1,3,3,3-Hexafluoroisopropanol as a Remarkable Medium for Atroposelective Sulfoxide-Directed Fujiwara-Moritani Reaction with Acrylates and Styrenes
Dherbassy, Quentin,Schwertz, Geoffrey,Chess, Matthieu,Hazra, Chinmoy Kumar,Wencel-Delord, Joanna,Colobert, Franoise
, p. 1735 - 1743 (2016)
Axially chiral biaryls are ubiquitous structural motifs of biologically active molecules and privileged ligands for asymmetric catalysis. Their properties are due to their configurationally stable axis, and therefore, the control of their absolute configuration is essential. Efficient access to atropo-enantioenriched biaryl moieties through asymmetric direct C-H activation, by using enantiopure sulfoxide as both the directing group (DG) and chiral auxiliary, is reported. The stereoselective oxidative Heck reactions are performed in high yields and with excellent atropo-stereoselectivities. The pivotal role of 1,1,1,3,3,3-hexafluoropropanol (HFIP) solvent, which enables a drastic increase in yield and stereoselectivity of this transformation, is evidenced and investigated. Finally, the synthetic usefulness of the herein disclosed transformation is showcased because the traceless character of the sulfoxide DG allows straightforward conversions of the newly accessed, atropopure sulfoxide-biaryls into several differently substituted axially chiral scaffolds.
Preparation method of hexafluoroisopropyl methyl ether
-
Paragraph 0051; 0052; 0060; 0061, (2019/06/30)
The invention discloses a preparation method of 1,1,1,3,3,3-hexafluoroisopropyl methyl ether. The preparation method includes reacting trifluoroacetate with formate to obtain 1,1,1,3,3,3-hexafluoroisopropanol, and applying a methylation reagent to the 1,1,1,3,3,3-hexafluoroisopropanol to obtain the 1,1,1,3,3,3-hexafluoroisopropyl methyl ether. The preparation method has the advantages that raw materials are cheap and easy to obtain, the preparation process is mild and the method is simple to operate.
Method for synthesizing hexafluoroacetone and method for synthesizing hexafluoroisopropanol
-
Paragraph 0059; 0060; 0061; 0062; 0063; 0064; 0065-0106, (2018/03/24)
The invention provides a method for synthesizing hexafluoroacetone and a method for synthesizing hexafluoroisopropanol. The method for synthesizing hexafluoroacetone comprises the following steps: generating first-step substitution reaction by virtue of hexachloroacetone and hydrogen fluoride under the effect of a first catalyst so as to generate a first product, wherein the temperature of the first-step substitution reaction is 70-80 DEG C, and the first catalyst is a trivalent chromium compound; and generating second-step substitution reaction by virtue of the first product and hydrogen fluoride under the effect of a second catalyst so as to generate hexafluoroacetone, wherein the temperature of the second-step substitution reaction is 350-400 DEG C, and the second catalyst is a trivalent chromium compound. The method for synthesizing hexafluoroisopropanol comprises the steps of carrying out all steps of the method for synthesizing hexafluoroacetone, and further carrying out reductive hydrogenation. According to the two methods, the problem that carbon is easily generated in a traditional process is solved.
Nickel N-heterocyclic carbene catalyzed C-C bond formation: A new route to aryl ketones
Gu, Li-Jun,Jin, Cheng,Zhang, Hong-Tao
supporting information, p. 8741 - 8744 (2015/06/08)
A novel nickel N-heterocyclic carbene catalyzed cross-coupling reaction of aryl aldehydes with boronic esters for the synthesis of aryl ketones was developed. This reaction provides a mild, practical method toward aryl ketones, which are versatile intermediates and building blocks in organic synthesis.