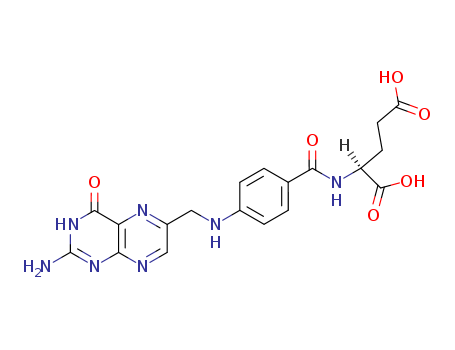
Folic acid literature
-
Uyeo et al.
, p. 5339 (1950)
-
Reaction of Imidazole and 2-Methylimidazole with Copper(II) Salts and Certain Acids
Il’ina, K. A.,Kozik, V. V.,Skorik, N. A.
, p. 1714 - 1721 (2021/11/24)
Abstract: Composition of biligand compounds Cu(C3H4N2, C4H6N2)x(Formula Presented.)2· nH2O obtained by reaction of aqueous suspensions of prepared poorly so
Method for preparing folic acid by virtue of micro-channel reaction (by machine translation)
-
Paragraph 0058-0060, (2020/12/14)
The invention belongs to the technical field of chemical synthesis of drugs, and relates to a synthesis method for preparing folic acid through a microchannel reactor. An intermediate 6 is prepared from cyanoethyl acetate as a raw material by one-step continuous operation, and the folic acid bulk drug is prepared through one-step reaction of the intermediate 6 and L - glutamate. The synthesis method uses the microchannel reactor to prepare the folic acid intermediate 2,triamino -4 - hydroxypyrimidine and folic acid, is safe and environment-friendly, and ensures the tasteless system. The method guarantees that the operation is simple and feasible, the solvent consumption is greatly reduced, 2,triamino -4 - hydroxyl pyrimidine yield and purity are obviously improved. (by machine translation)
Preparation method of 2,4,5-triamino-6-hydroxypyrimidine sulfate and folic acid
-
Paragraph 0098-0121, (2019/04/10)
The invention relates to a preparation method of 2,4,5-triamino-6-hydroxypyrimidine sulfate and folic acid. The preparation method comprises the following steps: enabling cyanoacetate and sodium nitrite to be subjected to reaction under the action of an organic solvent and/or an inorganic solvent and an acidic substance to obtain 2-oximidocyanoacetate; then, enabling the 2-oximidocyanoacetate andguanidine hydrochloride to be subjected to reaction under an alkaline condition to obtain 2,4-diamino-5-isonitroso-6-oxopyrimidine; enabling the 2,4-diamino-5-isonitroso-6-oxopyrimidine and hydrogen gas to be subjected to reaction under an alkaline condition by means of action of Pd/C to obtain 2,4,5-triamino-6-hydroxypyrimidine, and adding sulfuric acid to adjust the pH value to obtain 2,4,5-triamino-6-hydroxypyrimidine sulfate; and then, adding trichloroacetone and N-(4-aminobenzoyl)-L-glutamic acid to be subjected to reaction in a buffer solution under the action of a catalyst molecular sieve so as to obtain the folic acid. Based on the process route, the invention explores the influences of different reaction steps and refining conditions on the preparation route of 2,4,5-triamino-6-hydroxypyrimidine sulfate and the folic acid so as to reduce the wastewater pollution while increasing the yield.
Heteropolyacid catalyzed safe and green folic acid synthesis method
-
Paragraph 0032; 0056; 0067-0075, (2019/10/01)
The invention belongs to the technical field of pharmaceutical chemistry synthesis, and relates to a heteropolyacid catalyzed safe and green folic acid synthesis method. The folic acid synthesis method comprises the following steps that (1), acrolein, heteropoly acid, 2,5,6-triamino-4-hydroxypyrimidine, p-aminobenzoate and alcoholic solvents are added into a reactor, stirring and warming are conducted, a reaction is conducted, material cooling is conducted after the reaction is finished, filtration is conducted, a filter cake is washed with the solvents, filtrate and washing liquid are combined, activated carbon is used for decoloration, the solvents are dried through distillation after extraction filtration is conducted, and a yellowish solid intermediate 6 is obtained; (2), sodium hydrogen glutamate and the intermediate 6 are dissolved into an alcohol-water mixed solution, stirring and a temperature reaction are conducted, after the reaction is finished, material cooling and coolingare conducted, heat preservation and crystallization are conducted, extraction filtration is conducted, the filter cake is washed with water, a crude folic acid product is obtained, and folic acid isobtained through refining. According to the synthesis method, acrolein which is low in price and easy to obtain is used as a raw material, the reaction is complete and rapid, no residue exists, it isguaranteed that the system is tasteless, and the yield and purity are obviously increased.