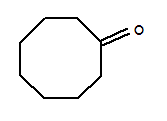
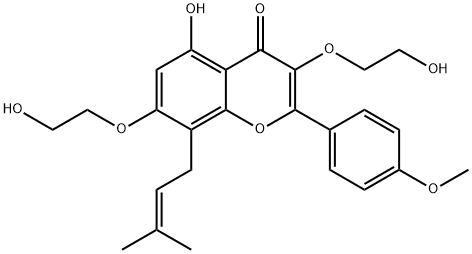
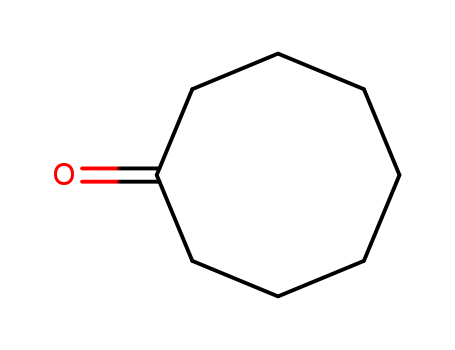Cyclooctanone literature
Oxidations of secondary alcohols to ketones using easily recyclable bis(trifluoroacetate) adducts of fluorous alkyl iodides, CF3(CF 2)n-1I(OCOCF3)2
Tesevic, Verona,Gladysz
, p. 7433 - 7440 (2006)
Reactions of commercial fluorous alkyl iodides RfnI (1-R fn; Rfn = CF3(CF2)n-1; n = 7, 8, 10, 12) with 80% H2O2 and trifluoroacetic anhydride give RfnI(OCOC
Manganese Porphyrins Supported on Montmorillonite as Hydrocarbon Mono-oxygenation Catalysts: Particular Efficacy for Linear Alkane Hydroxylation
Barloy, Laurent,Battioni, Pierrette,Mansuy, Daniel
, p. 1365 - 1367 (1990)
A supported Mn-porphyrin catalyst has been prepared by immobilisation of the tetracationic Mn Cl4+ on montmorillonite, and is found to be efficient for alkene epoxidation and alkane hydroxylation by PhIO, with a higher ability to oxidize alkanes, and in particular short linear alkanes, than corresponding homogeneous or silica-supported Mn-porphyrin catalysts.
Metal complexes of a tetraazacyclotetradecane bearing highly fluorinated tails: New catalysts for the oxidation of hydrocarbons under fluorous biphasic conditions
Pozzi, Gianluca,Cavazzini, Marco,Quici, Silvio,Fontana, Simonetta
, p. 7605 - 7608 (1997)
The commercially available macrocycle tetraazacyclotetradecane (cyclam) has been converted into the fluorocarbon soluble ligand 1 by N-functionalization with R(F)CH2OCH2CH2OTs 3, wherein R(F) is a (per)fluorooxyalkylenic c
Nonheme iron mediated oxidation of light alkanes with oxone: Characterization of reactive oxoiron(IV) ligand cation radical intermediates by spectroscopic studies and DFT calculations
Tse, Chun-Wai,Chow, Toby Wai-Shan,Guo, Zhen,Lee, Hung Kay,Huang, Jie-Sheng,Che, Chi-Ming
, p. 798 - 803 (2014)
The oxidation of light alkanes that is catalyzed by heme and nonheme iron enzymes is widely proposed to involve highly reactive {FeViO} species or {FeIViO} ligand cation radicals. The identification of these high-valent iron species and the development of an iron-catalyzed oxidation of light alkanes under mild conditions are of vital importance. Herein, a combination of tridentate and bidentate ligands was used for the generation of highly reactive nonheme {FeiO} species. A method that employs [Fe III(Me3tacn)(Cl-acac)Cl]+ as a catalyst in the presence of oxone was developed for the oxidation of hydrocarbons, including cyclohexane, propane, and ethane (Me3tacn=1,4,7-trimethyl-1,4,7- triazacyclononane; Cl-acac=3-chloro-acetylacetonate). The complex [Fe III(Tp)2]+ and oxone enabled stoichiometric oxidation of propane and ethane. ESI-MS, EPR and UV/Vis spectroscopy, 18O labeling experiments, and DFT studies point to [Fe IV(Me3tacn)({Cl-acac}.+)(O)]2+ as the catalytically active species. Highly reactive {FeiO} intermediates, such as [Fe(Tp)2(O)]+ or complex I (see Scheme), are likely to be involved in the oxidation of propane and ethane with oxone that is either mediated by [FeIII(Tp)2]+ (1) or catalyzed by iron complex 2. The cationic intermediate I features an {FeiO} moiety and is stabilized by a combination of tridentate and bidentate ligands. Copyright
-
Friess,Boekelheide
, p. 4145 (1949)
-
Transition metal-substituted polyoxometalates supported on MCM-41 as catalysts in the oxidation of cyclohexane and cyclooctane with H2O2
Jatupisarnpong, Jirarot,Trakarnpruk, Wimonrat
, p. 152 - 153 (2012)
The Keggin type polyoxometalates (Bu4N)4HPW11CoO39, (Bu4N)4PW11FeO39, (Bu4N)4HPW11CuO39 and (Bu4N)4PW11VO40 have been synthesized and supported on MCM-41 to be used as catalysts for the solvent-free oxidation of cyclohexane and cyclooctane with H2O2.
Novel iron porphyrin-alkanethiolate complex with intramolecular NH···S hydrogen bond: Synthesis, spectroscopy, and reactivity [3]
Suzuki, Noriyuki,Higuchi, Tsunehiko,Urano, Yasuteru,Kikuchi, Kazuya,Uekusa, Hidehiro,Ohashi, Yuji,Uchida, Takeshi,Kitagawa, Teizo,Nagano, Tetsuo
, p. 11571 - 11572 (1999)
-
Alkane hydroxylation by a nonheme iron catalyst that challenges the heme paradigm for oxygenase action
Company, Anna,Gomez, Laura,Gueell, Mireia,Ribas, Xavi,Luis, Josep M.,Que Jr., Lawrence,Costas, Miquel
, p. 15766 - 15767 (2007)
A nonheme iron catalyst catalyzed stereoselective oxidation of alkanes with H2O2 with remarkable efficiency and exhibiting an unprecedented high incorporation of water into the oxidized products. The present results challenge the canonical description of oxygenases, the standard oxo-hydroxo tautomerism that applies to heme systems and serves as a precedent for alternative pathways for the oxidation of hydrocarbons at nonheme iron oxygenases. Copyright
Iron catalysis for in situ regeneration of oxidized cofactors by activation and reduction of molecular oxygen: A synthetic metalloporphyrin as a biomimetic NAD(P)H oxidase
Maid, Harald,Boehm, Philipp,Huber, Stefan M.,Bauer, Walter,Hummel, Werner,Jux, Norbert,Groeger, Harald
, p. 2397 - 2400 (2011)
(Figure Presented) An enzyme substitute: A synthetic FeIII porphyrin was used as a catalyst for the activation and reduction of O 2 into H2O with the cofactor NAD(P)H in aqueous solution. The catalyst is compatible with different preparative enzymatic oxidation reactions. Thus, a new method is provided for the in situ regeneration of the oxidized cofactor NAD(P)+ with help from a nonenzymatic, synthetic catalyst (see scheme).
-
Blicke et al.
, p. 5418 (1953)
-
Reaction of aldehydes with the H5PV2Mo10O40 polyoxometalate and cooxidation of alkanes with molecular oxygen
Khenkin, Alexander M.,Rosenberger, Avi,Neumann, Ronny
, p. 82 - 91 (1999)
The oxidation of alkalies with molecular oxygen using aldehydes as reducing agents (aldehydes are cooxidized) was studied using the α-H5PV2Mo10O40 polyoxometalate as catalyst. Emphasis was placed on the initiation of the radical chain reaction by investigation of the aldehyde-polyoxometalate interaction. Using 31P NMR and ESR spectroscopy one could differentiate between the reactivity of the five inseparable isomers of α-H5PV2Mo10O40. Contrary to previous belief, the 1,11 isomer with vanadium in distal positions is the most abundant. The 31P NMR and ESR spectra supported by UV-vis absorption-time profiles of the reduction of α-H5PV2Mo10O40 indicated that isomers with vanadium in vicinal positions were most kinetically viable in the alkane oxidation. Addition of isobutyraldehyde to α-H5PV2Mo10O40 gave in the 51V NMR spectrum a new downfield peak attributed to the formation of an aldehyde-polyoxometalate intermediate. The alkane/aldehyde/O2 oxidizing system was found to be quite effective and selective for ketone formation. Reaction probes indicated that acyl peroxo radicals were the active oxidizing intermediates. Five pathways for its reaction were identified: chain propagation, alkane oxidation, decomposition to form oxygen, decomposition to acyl oxo radicals leading to CO2 and ketone, and capture and inhibition by the polyoxometalate.
Selective Homogeneous Catalytic Oxidation of Olefins using Oxygen/Hydrogen Mixtures: Oxygen Atom Transfer from an Iridium Hydroperoxide
Atlay, Mark T.,Preece, Michael,Strukul, Giorgio,James, Brian R.
, p. 406 - 407 (1982)
An iridium(III) hydride complex in solution catalyses the O2-co-oxidation of cyclo-octene and H2 to cyclo-octanone and water, respectively, via a hydroperoxide intermediate.
Templating an N-heterocyclic carbene (NHC)-cyclometalated Cp?IrIII-based oxidation precatalyst on a pendant coordination platform: Assessment of the oxidative behavior via electrochemical, spectroscopic and catalytic probes
Gupta, Suraj K.,Choudhury, Joyanta
, p. 1233 - 1239 (2015)
The coordination of metalloligands to derive modified properties of the metal functionality is one of the interesting strategies practiced in materials chemistry and catalysis. In this work, a pendant terpyridine ligand has been utilized for templating a Cp?IrIII(NHC)-based (NHC = N-heterocyclic carbene) oxidation precatalyst to assess its modified oxidative behavior via electrochemical, UV-vis spectroscopic, and catalytic probes. These studies suggested that the coordination-template enhances the electron-deficiency at the IrIII redox center and affects the nature of the oxidized high-valent Ir-oxo species during chemical oxidation. Moreover, both the premodified and postmodified Cp?IrIII(NHC)-based complexes were found to be equally efficient in catalytic sp3 C-H oxidation reactions with NaIO4 as a mild sacrificial oxidant.
Oxidation of Alkanes by Dioxygen Catalysed By Photoactivated Iron Porphyrins
Maldotti, A.,Bartocci, C.,Amadelli, R.,Polo, E.,Battioni, P.,Mansuy, D.
, p. 1487 - 1489 (1991)
Cycloalkanes were oxidized by O2 itself under mild conditions (22 deg C; 200 Torr of O2) in the presence of catalytic amounts of a polyhalogenated porphyrin-iron(III)-hydroxo complex irradiated with light of wavelength between 350 and 450 nm; these oxidations occurred without consumption of a reducing agent, selectively transformed cyclohexane into cyclohexanone under appropriate conditions (about 0.2 turnover per min), and did not involve FeV=O active species but, more probably, iron-alkylperoxo intermediates.
Simple soluble Bi(iii) salts as efficient catalysts for the oxidation of alkanes with H2O2
Rocha, Bruno G. M.,Kuznetsov, Maxim L.,Kozlov, Yuriy N.,Pombeiro, Armando J. L.,Shul'Pin, Georgiy B.
, p. 2174 - 2187 (2015)
A simple catalytic system based on a soluble bismuth(iii) salt, Bi(NO3)3/H2O2/HNO3/CH3CN + H2O, exhibits pronounced activity towards the homogeneous oxidation of inert alkanes with the yield of oxygenate products up to 32% and TON up to 112. The experimental selectivity parameters and kinetic data together with theoretical DFT calculations indicate that the reaction occurs via a free radical mechanism involving the formation of the HO radicals which directly react with alkane molecules. The mechanism of the HO generation (which is the rate limiting step of the whole process) includes the substitution of a water ligand for H2O2 in the initial aqua complex [Bi(H2O)8]3+, hydrolysis of the coordinated H2O2, second H2O-for-H2O2 substitution and the homolytic HO-OH bond cleavage in complex [Bi(H2O)4(H2O2)(OOH)]2+ (6). The relatively low overall activation energy for this process (ca. 20 kcal mol-1) is accounted for by the high lability and acidity of the Bi aqua complexes and tremendous activation of coordinated H2O2 in 6 towards homolysis. This journal is
Efficient catalytic cycloalkane oxidation employing a "helmet" phthalocyaninato iron(III) complex
Brown, Elizabeth S.,Robinson, Jerome R.,McCoy, Aaron M.,McGaff, Robert W.
, p. 5921 - 5925 (2011)
We have examined the catalytic activity of an iron(III) complex bearing the 14,28-[1,3-diiminoisoindolinato]phthalocyaninato (diiPc) ligand in oxidation reactions with three substrates (cyclohexane, cyclooctane, and indan). This modified metallophthalocyaninato complex serves as an efficient and selective catalyst for the oxidation of cyclohexane and cyclooctane, and to a far lesser extent indan. In the oxidations of cyclohexane and cyclooctane, in which hydrogen peroxide is employed as the oxidant under inert atmosphere, we have observed turnover numbers of 100.9 and 122.2 for cyclohexanol and cyclooctanol, respectively. The catalyst shows strong selectivity for alcohol (vs. ketone) formation, with alcohol to ketone (A/K) ratios of 6.7 and 21.0 for the cyclohexane and cyclooctane oxidations, respectively. Overall yields (alcohol + ketone) were 73% for cyclohexane and 92% for cyclooctane, based upon the total hydrogen peroxide added. In the catalytic oxidation of indan under similar conditions, the TON for 1-indanol was 10.1, with a yield of 12% based upon hydrogen peroxide. No 1-indanone was observed in the product mixture.
Structural studies and physico-chemical properties of new oxodiperoxomolybdenum complexes with nicotinic acid
Szymańska, Anna,Nitek, Wojciech,Mucha, Dariusz,Karcz, Robert,Pamin, Katarzyna,Po?towicz, Jan,?asocha, Wies?aw
, p. 39 - 46 (2013)
Three new oxodiperoxomolybdenum complexes with nicotinic acid have been synthesized and characterized with the use of single-crystal analysis, XRPD versus temperature, TG/DSC, SEM and IR spectroscopy. The investigated compounds are: 1 - (NH4)2·[MoO(O2) 2.N-nicO]2, 2 - (nicH)2·[MoO(O 2)2.NnicO]2·2(H2O), 3 - MoO(O2)2(H2O)nicH; (N-nicO, nicH - denote nicotinic acid N-oxide and protonated nicotinic acid, respectively). Compounds 1, 2 are salts of the same acid with cyclic dinuclear anions, whereas compound 3 can be regarded as an inner salt. Compounds 1-3 are stable in ambient conditions, the respective percentages of oxygen in peroxo groups are 19.21%, 14.06% and 20.19%, respectively. Above 125 °C peroxo compounds 1-3 decompose, forming nanometric MoO2 and MoO3. TG/DSC curves reveal clear effects connected with the release of oxygen and strong exothermic effects connected with final loss of organic components. All the investigated complexes were found to be active in the oxidation of cyclooctane to cyclooctanone and cyclooctanol with molecular oxygen.
V(iv), Fe(ii), Ni(ii) and Cu(ii) complexes bearing 2,2,2-tris(pyrazol-1-yl)ethyl methanesulfonate: Application as catalysts for the cyclooctane oxidation
Silva, Telma F. S.,Rocha, Bruno G. M.,Guedes Da Silva, M. Fátima C.,Martins, Luísa M. D. R. S.,Pombeiro, Armando J. L.
, p. 528 - 537 (2016)
Water-soluble compounds [VOCl2{CH3SO2OCH2C(pz)3}] (pz = pyrazol-1-yl) 1, [FeCl2{CH3SO2OCH2C(pz)3}] 2, [NiCl2{CH3SO2OCH2C(pz)3}] 3 and [Cu{CH3SO2OCH2C(pz)3}2](OTf)24 were obtained by reactions between the corresponding metal salts and 2,2,2-tris(pyrazol-1-yl)ethyl methanesulfonate, CH3SO2OCH2C(pz)3. They were isolated as air-stable solids and fully characterized by IR, FTIR, NMR (for 2), EPR (for 1), ESI-MS(+/-), elemental analysis and (for 4) single-crystal X-ray diffraction. In all, half- (1-3) or full-sandwich (4), compounds the C-scorpionate ligand shows the N,N,N-coordination mode. 3 and 4 appear to provide the first examples of a Ni(ii) and a full-sandwich Cu(ii) compound respectively, bearing that scorpionate ligand. Compound 3 is the first Ni(ii) tris(pyrazol-1-yl)methane type complex to be applied as catalyst for the oxidation of alkanes. Compounds 1-4 exhibit catalytic activity for the peroxidative (with aq. H2O2) oxidation, in water/acetonitrile medium and under mild homogeneous conditions, of cyclooctane to the corresponding alcohol and ketone (yields up to ca. 27%). The effect of the presence of additives, such as nitric acid or pyridine, was studied.
Cobalt(II) Catalyzed Biomimetic Oxidation of Hydrocarbons in the Presence of Dioxygen and 2-Methylpropanal
Punniyamurthy, T.,Kalra, Swinder Jeet Singh,Iqbal, Javed
, p. 8497 - 8500 (1995)
Cobalt(II) Schiff base complex 1 catalyses the oxidation of aliphatic and aromatic hydrocarbons in the presence of 2-methylpropanal under 1 atmosphere of dioxygen to give corresponding ketones and alcohols.
-
Stoll,Rouve
, p. 1570,1578, 1579, 1582 (1944)
-
Oxidation of cycloalkanes with molecular oxygen in the presence of salen metallocomplexes in thermomorphic conditions
Pamin, Katarzyna,Pozzi, Gianluca,Tabor, Edyta,Bukowski, Wiktor,Po?towicz, Jan
, p. 102 - 105 (2013)
The oxidation of cycloalkanes with molecular oxygen, catalyzed by two groups of metallosalen complexes, was studied. The first group consisted of salen complexes with different metals such as Mn, Fe, Co, Ni, Cu, Zn, while the second group was composed of manganese salen complexes with different substituents (t-butyl electron-donating substituents and/or electron-withdrawing perfluoroalkyl substituents). Mn, Fe and Co salen complexes are the most active catalysts, while Ni, Cu and Zn salen complexes are far less efficient. The introduction of t-butyl electron-donating substituents into Mnsalen complex increases the catalytic activity and catalysts solubility in the reaction mixture. The introduction of perfluoroalkyl electron-withdrawing substituents enhances the catalytic activity and renders the solubility of the catalyst temperature dependent (thermomorphic behaviour), thus allowing one to recover them easily after the reaction by simply cooling the system to room temperature. The synthesis of two new manganese salen complexes with perfluoroalkyl substituents was elaborated.
Hydrogen Peroxide Oxygenation of Alkanes catalysed by Manganese(III)-tetraarylporphyrins: the Remarkable Co-catalytic Effect of Lipophilic Carboxylic Acids and Heterocyclic Bases
Banfi, Stefano,Maiocchi, Alessandro,Moggi, Alberto,Montanari, Fernando,Quici, Silvio
, p. 1794 - 1796 (1990)
Oxygenation of alkanes promoted by 30percent H2O2 and catalysed by chemically robust manganese(III)-tetraaryl porphyrins 2-4 is strongly accelerated by addition of small amounts of lipophilic carboxylic acids and lipophilic heterocyclic bases; cycloalkanes are converted into mixtures of alcohols and ketones at a rate of up to 125 turnovers min-1 in CH2Cl2-H2O solution at 0 deg C.
Rhodium mediated C-H Bond functionalisation leading to carboxylate: Derivatives
Tejel, Cristina,Del Rio, M. Pilar,Lopez, Jose A.,Ciriano, Miguel A.
, p. 11261 - 11265 (2010)
Selective functionalisation of 15-cyclooctadiene either to a carboxylic acid or to a γ-lactone is achieved by a novel sequence of reactions involving quantitative oxygenation with oxygen and C-C coupling with carbon monoxide (see graphic). The rhodium hydroxy-alkyl-allyl complex shown is the active intermediate that promotes the elimination of the organic compounds.
Oxidation of alkanes catalyzed by manganese(III) porphyrin in anionic liquid at room temprature
Li, Zhen,Xia, Chun-Gu,Xu, Chuan-Zhi
, p. 9229 - 9232 (2003)
Efficient oxidation of alkanes is achieved by using an electron-deficient manganese(III) porphyrin catalyst in combination with iodobenzene diacetate in an ionic liquid at room temperature; a high-valent manganese-oxo porphyrin complex (MnV=O) was considered as the reactive oxidation intermediate.
Polymer- And silica-supported iron BPMEN-inspired catalysts for C-H bond functionalization reactions
Feng, Yan,Moschetta, Eric G.,Jones, Christopher W.
, p. 3142 - 3152 (2014)
Direct catalytic C-H bond functionalization is a key challenge in synthetic chemistry, with many popular C-H activation methodologies involving precious-metal catalysts. In recent years, iron catalysts have emerged as a possible alternative to the more common precious-metal catalysts, owing to its high abundance, low cost, and low toxicity. However, iron catalysts are plagued by two key factors: the ligand cost and the low turnover numbers (TONs) typically achieved. In this work, two approaches are presented to functionalize the popular N1,N2-dimethyl-N1,N2-bis(pyridin-2-ylmethyl)-ethane-1,2-diamine (BPMEN) ligand, so that it can be supported on porous silica or polymer resin supports. Four new catalysts are prepared and evaluated in an array of catalytic C-H functionalization reactions by using cyclohexane, cyclohexene, cyclooctane, adamantane, benzyl alcohol, and cumene with aqueous hydrogen peroxide. Catalyst recovery and recycling is demonstrated by using supported catalysts, which allows for a modest increase in the TON achieved with these catalysts.
Efficient and selective oxidation of hydrocarbons with tert-butyl hydroperoxide catalyzed by oxidovanadium(IV) unsymmetrical Schiff base complex supported on γ-Fe2O3 magnetic nanoparticles
Samani, Mahnaz,Ardakani, Mehdi Hatefi,Sabet, Mohammad
, p. 1481 - 1494 (2022/01/22)
The catalytic activity of an oxidovanadium(IV) unsymmetrical Schiff base complex supported on γ-Fe2O3 magnetic nanoparticles, γ-Fe2O3@[VO(salenac-OH)] in which salenac-OH = [9-(2′,4′-dihydroxyphenyl)-5,8-diaza-4
Synthesis of Visible-Light–Activated Hypervalent Iodine and Photo-oxidation under Visible Light Irradiation via a Direct S0→Tn Transition
Matsuda, Yu,Matsumoto, Koki,Nagasawa, Sho,Nakajima, Masaya,Nemoto, Tetsuhiro
, p. 235 - 239 (2022/03/16)
Heavy atom-containing molecules cause a photoreaction by a direct S0→Tn transition. Therefore, even in a hypervalent iodine compound with a benzene ring as the main skeleton, the photoreaction proceeds under 365–400nm wavelength light, where UV-visible spectra are not observed by usual measurement method. Some studies, however, report hypervalent iodine compounds that strongly absorb visible light. Herein, we report the synthesis of two visible light-absorbing hypervalent iodines and their photooxidation properties under visible light irradiation. We also demonstrated that the S0→Tn transition causes the photoreaction to proceed under wavelengths in the blue and green light region.
Gold-Catalyzed Direct C(sp3)?H Acetoxylation of Saturated Hydrocarbons
Jo, Tae Geun,Klein, Johannes E. M. N.
, p. 4087 - 4091 (2021/08/25)
In this communication we report our studies towards the development of a gold-catalyzed direct acetoxylation of C(sp3)?H bonds. We achieve this through the use of the hypervalent iodine reagent PhI(OAc)2 in combination with a simple gold salt (HAuBr4) as the catalyst. Through a comparison of the reactivities of cyclooctane and adamantane we judge the reaction to proceed via hydride transfer. This is further substantiated through computational studies of the relative energies for the anions, radicals and cations derived from C?H bond cleavage of cyclooctane and adamantane relevant to the C?H cleaving step.
Nonheme Diiron Oxygenase Mimic That Generates a Diferric-Peroxo Intermediate Capable of Catalytic Olefin Epoxidation and Alkane Hydroxylation including Cyclohexane
Kaizer, József,Oloo, Williamson N.,Que, Lawrence,Szávuly, Miklós
, (2021/12/27)
Herein are described substrate oxidations with H2O2 catalyzed by [FeII(IndH)(CH3CN)3](ClO4)2 [IndH = 1,3-bis(2′-pyridylimino)isoindoline], involving a spectroscopically characterized (μ-oxo)(μ-1,2-peroxo)diiron(III) intermediate (2) that is capable of olefin epoxidation and alkane hydroxylation including cyclohexane. Species 2 also converts ketones to lactones with a decay rate dependent on [ketone], suggesting direct nucleophilic attack of the substrate carbonyl group by the peroxo species. In contrast, peroxo decay is unaffected by the addition of olefins or alkanes, but the label from H218O is incorporated into the the epoxide and alcohol products, implicating a high-valent iron-oxo oxidant that derives from O-O bond cleavage of the peroxo intermediate. These results demonstrate an ambiphilic diferric-peroxo intermediate that mimics the range of oxidative reactivities associated with O2-activating nonheme diiron enzymes.