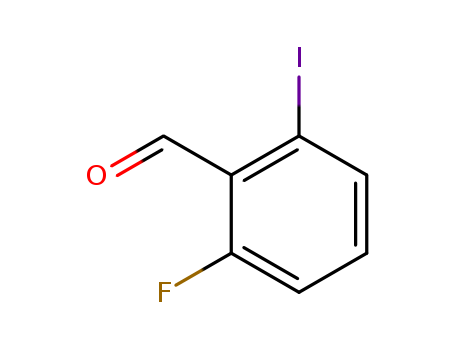
2-FLUORO-6-IODOBENZALDEHYDE literature
A benzo[b]thiophene-based selective type 4 S1P receptor agonist
Hur, Wooyoung,Rosen, Hugh,Gray, Nathanael S.
, p. 1 - 5 (2017)
S1P receptors (S1PR1-5) are a group of GPCRs activated by a high affinity binding with S1P that have important roles in the regulation of the immune system. A potent S1PR agonist FTY720 is an immunomodulator used to treat multiple sclerosis and several ‘second generation’ drugs are under clinical development. Subtype-selective agonists have been reported for each S1PR isotype, some of which are used as pharmacological tools for functional studies. Here we report the discovery and initial characterization of compound 5c, a benzo[b]thiophene amino carboxylate which exhibits potent and selective agonist activity for S1PR4. Compound 5c has an EC50= 200 nM as an agonist in GTPγ35S binding assay for S1PR4 and exhibits no activity against S1PR1,2,3,5. We confirmed its potent activity and decent S1PR subtype selectivity using biochemical and cellular assays.
Latent Nucleophilic Carbenes
Marchenko, Anatoliy,Koidan, Georgyi,Hurieva, Anastasiya,Shvydenko, Kostiantyn,Rozhenko, Alexander B.,Rusanov, Eduard B.,Kyrylchuk, Andrii A.,Kostyuk, Aleksandr
, p. 373 - 385 (2021/12/27)
Using DFT and ab initio calculations, we demonstrate that noncyclic formamidines can undergo thermal rearrangement into their isomeric aminocarbenes under rather mild conditions. We synthesized the silylformamidine, for which the lowest activation energy in this process was predicted. Experimental studies proved it to serve as a very reactive nucleophilic carbene. The reactions with acetylenes, benzenes, and trifluoromethane proceeded via insertion into sp, sp2, and spCH bonds. The carbene also reacted with the functional groups, such as CHO, COR, and CN at double or triple bonds, displaying high mobility of the trimethylsilyl group. The obtained silylformamidine can be considered as a latent nucleophilic carbene. It can be prepared in bulk quantities, stored, and used when the need arises. Calculation results predict similar behavior for some other silylated formamidines and related compounds.
Palladium-Catalyzed, Norbornene-Mediated, ortho-Amination ipso-Amidation: Sequential C-N Bond Formation
Whyte, Andrew,Olson, Maxwell E.,Lautens, Mark
, p. 345 - 348 (2018/01/27)
A palladium-catalyzed, norbornene-mediated ortho- and ipso-C-N bond-forming Catellani reaction is reported. This reaction proceeds through a sequential intermolecular amination followed by intramolecular cyclization of a tethered amide. The products, ortho-aminated dihydroquinolinones, were generated in moderate to good yields and are present in bioactive molecules. This work highlights the challenge of competing intra- vs intermolecular palladium-catalyzed processes.
F- Nucleophilic-Addition-Induced [3 + 2] Annulation: Direct Access to CF3-Substituted Indenes
Tang, Hai-Jun,Zhang, Yu-Feng,Jiang, Yi-Wen,Feng, Chao
, p. 5190 - 5193 (2018/09/12)
An efficient [3 + 2] annulation of (2,2-difluorovinyl)-2-iodoarenes and internal alkynes was developed for the synthesis of 1-(trifluoromethyl)-1H-indenes. The success of this strategy hinges upon a well-balanced process for the generation of two transient reactive species, specifically trifluoroethylsilver and alkenylpalladium intermediates, in the same molecule, as well as a smooth transmetalation step, which delicately joins together these two different metallic intermediates.