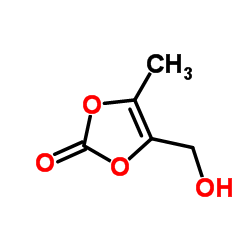
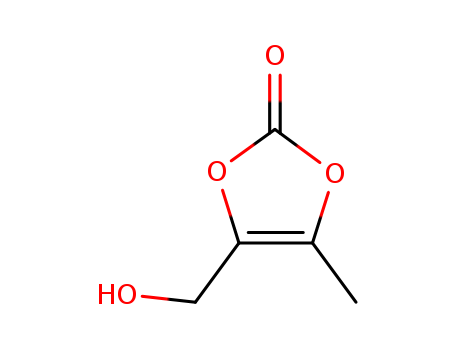
4-(hidroximetil)-5-metil-1,3-dioxol-2-ona literature
MONOETHYLENICALLY UNSATURATED MONOMERS AND USES THEREOF
-
, (2021/06/22)
The invention relates to a monoethylenically unsaturated monomer of formula (I), and to the use thereof for producing a polymer. The invention also relates to the polymer obtained by polymerising said monomer, and to the use thereof in a composition for producing coatings.
Preparation method of olmesartan medoxomil key intermediate
-
Paragraph 0026-0034, (2021/08/07)
The invention discloses a preparation method of an olmesartan medoxomil key intermediate, and belongs to the technical field of medicine synthesis. The key points of the technical scheme are as follows: triphosgene with relatively low toxicity is adopted to replace gaseous phosgene, so that the problems of storage and transportation are solved; and a molecular distillation technology is utilized to treat the crude product, so that the occurrence of a polymer at high temperature is avoided, and a high-yield product is obtained.
AN IMPROVED PROCESS FOR 4-(HYDROXYMETHYL)-5-METHYL-1,3-DIOXOL-2-ONE
-
Page/Page column 5; 7-8, (2021/04/17)
The present invention relates to an improved process for 4-(Hydroxymethyl)-5-methyl-1,3-dioxol-2-one (I). The process involves reaction of compound of formula (II) with sodium acetate in presence of catalytic amount of potassium iodide in dimethyl formamide solvent at 25-30°C to give 5-methyl-2-oxo-1,3-dioxol-4-yl)methyl acetate (IV) which was further Acid hydrolysed by IPA. HCl in Isopropyl alcohol solvent to yield 4-(hydroxymethyl)-5-methyl-1,3-dioxol-2-one (I).
Method for synthesizing 4 - (hydroxymethyl) -5 - methyl-[1, 3] dioxole -2 - ketone (by machine translation)
-
Paragraph 0009; 0022-0042, (2020/09/20)
The invention discloses a method for synthesizing 4 - (hydroxymethyl) -5 - methyl-[1, 3] dioxole -2 - ketone, and belongs to the technical field of chemical synthesis. The reaction equation in the synthesis process is as follows. By selecting proper solvent and hydrolysis medium and optimizing the dosage ratio between reactants, the reaction rate and yield are greatly improved, the reaction is more complete and thorough, the yield of the target product reaches 92%, and the purity of the target product reaches 97%. (by machine translation)