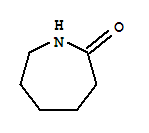
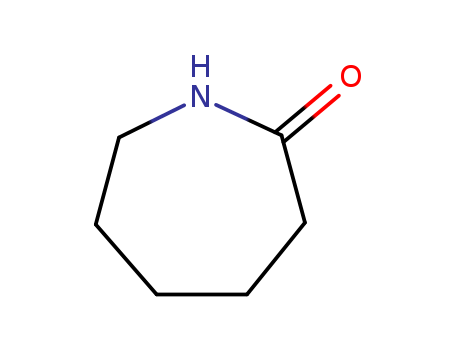
6-Caprolactam literature
Hierarchical silicalite-1 octahedra comprising highly-branched orthogonally-stacked nanoplates as efficient catalysts for vapor-phase Beckmann rearrangement
Chang, Albert,Hsiao, Hsu-Ming,Chen, Tsai-Hsiu,Chu, Ming-Wen,Yang, Chia-Min
, p. 11939 - 11942 (2016)
A triblock structure-directing agent was designed to synthesize hierarchical silicalite-1 octahedra comprising highly-branched, orthogonally-stacked and self-pillared nanoplates that exhibited excellent and stable activity for the vapor-phase Beckmann rearrangement of cyclic oximes and high lactam selectivity.
VAPOR-PHASE BECKMANN REARRANGEMENT OF CYCLOHEXANONE OXIME OVER SILICA-BORIA CATALYST PREPARED BY CHEMICAL VAPOR DEPOSITION METHOD
Sato, Satoshi,Sakurai, Hiroaki,Urabe, Kazuo,Izumi, Yusuke
, p. 277 - 278 (1985)
Cyclohexanone oxime was converted to ε-caprolactam in a high yield of 93percent at 250 deg over a silica-supported boron oxide catalyst which was prepared by means of chemical vapor deposition.This catalyst was much more efficient than silica-boria and alumina-boria which were obtained by the usual impregnation method.
Active Sites for the Liquid-Phase Beckmann Rearrangement of Cyclohexanone, Acetophenone and Cyclododecanone Oximes, Catalyzed by Beta Zeolites
Camblor,Corma,Garcia,Semmer-Herledan,Valencia
, p. 267 - 272 (1998)
The Beckmann rearrangement of oximes with different molecular sizes, i.e. cyclohexanone, cyclododecanone, and acetophenone oximes, has been studied in liquid phase at 130°C over a series of four Beta zeolites differing in the presence or absence of framework Al and internal silanol groups. When the zeolite does not contain framework Al and internal silanols, no appreciable conversion was observed. The catalyst having internal silanol groups but no framework Al exhibits oxime conversion, but the selectivity to the corresponding amide is low in some cases. In the Beta zeolite without silanol groups but containing framework Al, conversion and selectivity were found to be very high. This superior performance of Broensted acid sites, compared to silanol groups, shows that the results reported for the vapor phase reaction cannot be extrapolated when the reaction is performed in liquid phase. Finally, as could be anticipated according to the dimensions of the micropores, it is shown that H-Beta zeolites exhibit a much better catalytic performance than H-ZSM-5 zeolite for larger sized oximes.
Ammoximation of cyclohexanone by nitric oxide and ammonia: A One-step process for synthesis of polycaprolactam
Prasad,Vashisht, Seema
, p. 373 - 376 (1996)
Catalytic transformation of cyclohexanone by nitric oxide and ammonia to cyclohexanone oxime, its rearrangement to caprolactam, and its polymerization to polycaprolactam has been studied in the liquid phase over solid catalysts. The influence of various catalysts and process parameters on the oximation reaction is reported. A maximum yield of 37.78% with a selectivity of 80.95% for caprolactam could be achieved, over a synthetically prepared Al2O3-SiO2 catalyst, at a cyclohexanone : NO : NH3 molar ratio of 1:2.9:1.7 and a temperature of 348 K. In this process the production of oxime and its rearrangement to caprolactam occur simultaneously. The resulting caprolactam polymerises later to give a molecular weight ranging from 500 to 5000. A tentative mechanism for the reactions is suggested.
Porous aluminosilicate inorganic polymers (geopolymers): a new class of environmentally benign heterogeneous solid acid catalysts
Alzeer, Mohammad I.M.,MacKenzie, Kenneth J.D.,Keyzers, Robert A.
, p. 173 - 181 (2016)
Aluminosilicate inorganic polymers (geopolymers) were developed as a new class of cost-efficient, environmentally friendly, solid acid catalysts and their performance evaluated in a model liquid-phase Beckmann rearrangement reaction (cyclohexanone oxime to ε-caprolactam). The active sites were generated within the structure of the geopolymers by ion-exchange with NH4+ followed by thermal treatment. The effect of varying the starting composition on the textural and acidic properties of the geopolymer catalysts was studied and its influence on the catalytic activity was investigated. Catalytic performance was significantly improved by the use of post-synthetic treatments. No significant decrease in the yield of ε-caprolactam after recycling for five times suggesting that geopolymer-based catalysts are advantageous over supported catalysts which often lose their catalytic activity due to leaching of the active sites from the support. The catalytic activities obtained in this study are comparable, and sometimes superior, to other solid catalysts suggesting that geopolymers have a great potential as environmentally benign heterogeneous catalysts.
An Integrated Cofactor/Co-Product Recycling Cascade for the Biosynthesis of Nylon Monomers from Cycloalkylamines
Sarak, Sharad,Sung, Sihyong,Jeon, Hyunwoo,Patil, Mahesh D.,Khobragade, Taresh P.,Pagar, Amol D.,Dawson, Philip E.,Yun, Hyungdon
, p. 3481 - 3486 (2021)
We report a highly atom-efficient integrated cofactor/co-product recycling cascade employing cycloalkylamines as multifaceted starting materials for the synthesis of nylon building blocks. Reactions using E. coli whole cells as well as purified enzymes produced excellent conversions ranging from >80 and 95 % into desired ω-amino acids, respectively with varying substrate concentrations. The applicability of this tandem biocatalytic cascade was demonstrated to produce the corresponding lactams by employing engineered biocatalysts. For instance, ?-caprolactam, a valuable polymer building block was synthesized with 75 % conversion from 10 mM cyclohexylamine by employing whole-cell biocatalysts. This cascade could be an alternative for bio-based production of ω-amino acids and corresponding lactam compounds.
Amino-alcohol cyclization: Selective synthesis of lactams and cyclic amines from amino-alcohols
Pingen, Dennis,Vogt, Dieter
, p. 47 - 52 (2014)
By employing an amination catalyst, previously used in the direct synthesis of amines from alcohol with ammonia, n-amino-alcohols could be selectively cyclized to either the amide or the amine. By the addition of water, the amine could be produced as the major product whereas adding a sacrificial ketone as a hydrogen acceptor resulted in the amide as the major product. Without an additive a mixture of both the amine and the amide was observed. N-substituted amino-alcohols solely gave cyclic amines under these conditions. From 2-(n-alkanol) anilines the cyclic amines were produced, where the n-propanol derivative selectively formed quinoline as the major product.
-
Kelly,Matthews
, p. 2159 (1971)
-
Catalytic properties of WOx/SBA-15 for vapor-phase Beckmann rearrangement of cyclohexanone oxime
Bordoloi, Ankur,Halligudi
, p. 141 - 147 (2010)
WOx/SBA-15 nanocomposite materials with different WOx loadings were prepared by one step hydrothermal synthesis and used in the vapor-phase Beckmann rearrangement of cyclohexanone oxime to ε-caprolactam. The catalysts were thoroughly characterized by X-ray diffraction (XRD), sorption analysis, energy dispersive X-ray analysis (EDAX) and Raman spectroscopy. The acidities of the catalysts were estimated by ammonia temperature programmed desorption (NH3-TPD) and Fourier transform infrared studies of adsorbed pyridine (pyridine-FTIR). The optimum temperature for the Beckmann rearrangement was 350 °C. Using WOx/SBA-15(20) under the vapor-phase reaction conditions [temperature = 350 °C, WHSV = 0.6 h-1, oxime concentration = 2.5% (w/w) in MeOH] gave 79% cyclohexanone oxime conversion with 93%, ε-caprolactam selectivity. The ε-caprolactam selectivity was found to be dependent on temperature and space velocity. A correlation has been made between the rearrangement activity and acidity and the structural properties of the catalysts.
-
Kobayashi
, p. 3467,3470 (1973)
-
Weil-defined N-heterocyclic carbene based ruthenium catalysts for direct amide synthesis from alcohols and amines
Zhang, Yao,Chen, Cheng,Ghosh, Subhash Chandra,Li, Yongxin,Hong, Soon Hyeok
, p. 1374 - 1378 (2010)
Well-defined N-heterocyclic carbene based ruthenium, complexes were developed as highly active catalysts for direct amide synthesis from alcohols and amines. A catalytic amount of a base such, as KO1Bu was essential to initiate the catalytic cycle. Activity of the Ru complexes was comparable with the reported in situ Ru catalysts. These catalysts provided mechanistic insight suggesting a Ru hydride species as an active catalytic intermediate. The generation of the Ru hydride was critical for the amidation of free aldehydes.
Construction of tertiary chiral centers by Pd-catalyzed asymmetric allylic alkylation of prochiral enolate equivalents
Kita, Yusuke,Numajiri, Yoshitaka,Okamoto, Noriko,Stoltz, Brian M.
, p. 6349 - 6353 (2015)
Abstract The palladium-catalyzed decarboxylative allylic alkylation of enol carbonates derived from lactams and ketones is described. Employing these substrates with an electronically tuned Pd catalyst system trisubstituted chiral centers are produced. These stereocenters have been previously challenging to achieve using Pd complex/chiral P-N ligand systems.
One-pot conversion of lysine to caprolactam over Ir/H-Beta catalysts
Sebastian, Joby,Zheng, Mingyuan,Jiang, Yu,Zhao, Yu,Wang, Hua,Song, Zhendong,Li, Xinsheng,Pang, Jifeng,Zhang, Tao
, p. 2462 - 2468 (2019)
Amino acid lysine could serve as an ideal bio-based feedstock for the synthesis of caprolactam (CPL), which is currently a petroleum-derived monomer. Herein, we report the one-pot conversion of l-lysine to CPL via hydrogenolysis over bifunctional metal supported catalysts. Among the various hydrogenation metals and different supports, the combination of Ir and HB zeolite gave the best performance. Under optimal conditions, a 30% yield of CPL from l-lysine and a 58% yield from the reaction intermediate α-dimethyl amino caprolactam (DMAC) were obtained over a 2Ir/HB-124 catalyst at 250 °C in an autoclave or fixed-bed reactor. The reaction solvent dramatically affected the reaction selectivity, and methanol was found to be the best due to its unique contribution towards the formation of α-dimethyl amino caprolactam (DMAC) as well as the following C-N breakage of the C-N(CH3)2 bond. The acid sites on the catalyst accelerate lactam formation, and the synergy between the acid sites and hydrogenation sites favours C-N bond hydrogenolysis to produce CPL. Besides the acidity, the large pore size of HB is able to accommodate big reaction intermediate molecules inside the pores further ensures the superior performance of Ir/HB. The reaction route was identified, i.e., l-lysine first undergoes cyclization and N-methylation to DMAC, and then C-N(CH3)2 bond hydrogenolysis to form CPL. The Ir/HB catalyst has reasonably good stability and high selectivity, making this one-pot conversion process a novel and environmentally benign way of producing CPL from easily available renewable feedstocks.
Preparation of SiO2-Al2O3 and its supported Co3O4 Catalysts for nitrosation of cyclohexane
Liu, Pingle,Xie, Huan,You, Kuiyi,Hao, Fang,Li, Xiaofeng,Luo, He'An
, p. 150 - 154 (2010)
Amorphous SiO2-Al2O3 was prepared by sol-gel method and cobalt oxide supported on SiO2-Al 2O3 was prepared by impregnation method. These materials were characterized by BET, XRD, TPD and FT
A novel reaction of N-phenylthiocaprolactam: The α-sulfenylation of ketones under mild conditions
Foray, Gabriela,Penenory, Alicia B.,Rossi, Roberto A.
, p. 2035 - 2038 (1997)
N-phenylthiocaprolactam (2) reacts with the enolate anions of aliphatic, aromatic or cyclic ketone 1a-e, to give the corresponding α-phenylthioketones 3a-e. This reaction proceeds with high yields of monosulphenylation (80-97%) in DMSO under mild conditions (potassium ter-butoxide, 25°C, 10 min).
Lysine-based functional blocked isocyanates for the preparation of polyurethanes provided with pendant side groups
Yin, Jie,Wildeman, Jur,Loontjens, Ton
, p. 2036 - 2049 (2015)
This article describes a methodology to prepare polyurethanes (PUs), decorated with pendant (bio)functional side groups, by polymerizing (bio)functionalized blocked diisocyanates with polyols. Caprolactam blocked lysine diisocyanate methyl ester (BLDI-OMe) was prepared in high yields, by reacting the methyl ester of lysine with carbonyl biscaprolactam. In the absence of a catalyst, the polymerization of BLDI-OMe with polycaprolactone and polytetrahydrofuran resulted in strictly linear PUs due to the high selective reactivity of the blocked isocyanates (BIs). Although the ester appeared to be less reactive, we found hydrolyzing conditions for the ester, without affecting the BIs. The free acid groups were converted into a N-hydroxysuccinimide (NHS) activated ester, which was a versatile intermediate for further functionalization. After having demonstrated that model amines were able to substitute NHS without effecting the BIs groups, the same chemistry was used to couple biotin, giving a biotin functional caprolactam blocked lysine diisocyanate. The polymerization with polyols afforded the corresponding biotin-functional PUs.
Aerobic Oxidation of Cyclic Amines to Lactams Catalyzed by Ceria-Supported Nanogold
Dairo, Taiwo O.,Nelson, Nicholas C.,Slowing, Igor I.,Angelici, Robert J.,Woo, L. Keith
, p. 2278 - 2291 (2016)
Abstract: The oxidative transformation of cyclic amines to lactams, which are important chemical feedstocks, is efficiently catalyzed by CeO2-supported gold nanoparticles (Au/CeO2) and Aerosil 200 in the presence of an atmosphere of O2. The complete conversion of pyrrolidine was achieved in 6.5?h at 160 °C, affording a 97 % yield of the lactam product 2-pyrrolidone (γ-butyrolactam), while 2-piperidone (δ-valerolactam) was synthesized from piperidine (83 % yield) in 2.5?h. Caprolactam, the precursor to the commercially important nylon-6, was obtained from hexamethyleneimine in 37 % yield in 3?h. During the oxidation of pyrrolidine, two transient species, 5-(pyrrolidin-1-yl)-3,4-dihydro-2H-pyrrole (amidine-5) and 4-amino-1-(pyrrolidin-1-yl)butan-1-one, were observed. Both of these compounds were oxidized to 2-pyrrolidone under catalytic conditions, indicating their role as intermediates in the reaction pathway. In addition to the reactions of cyclic secondary amines, Au/CeO2 also efficiently catalyzes the oxidation of N-methyl cyclic tertiary amines to the corresponding lactams at 80 and 100 °C. Graphical Abstract: [Figure not available: see fulltext.]
Ring contraction of N-chlorolactams, a novel rearrangement
Drouin, Alexandre,Lessard, Jean
, p. 4285 - 4288 (2006)
Upon photolysis in methylene chloride at -78 °C, different N-chlorolactams underwent a novel ring contraction to the corresponding carbamoyl chlorides, which were converted to the methyl carbamates. The rearrangement is 100% stereoselective, occurring with retention of configuration at the migrating carbon center. The yields of isolated carbamates ranged from 40% to 57%, the other product being the parent lactam, 18% to 38%.
Effective depolymerization of nylon-6 in wet supercritical hydrocarbons
Kaiso, Kouji,Sugimoto, Tsunemi,Kashiwagi, Kohichi,Kamimura, Akio
, p. 370 - 371 (2011)
Treatment of nylon-6 with supercritical toluene in the presence of small amounts of water resulted in an effective conversion of polyamide to give ε-caprolactam in quantitative yield. The presence of a small amount of water is critical for the progress of the reaction; completely anhydrous conditions failed to achieve depolymerization. ε-Caprolactam was readily isolated after the removal of toluene under reduced pressure. The present method can serve as a useful treatment for the effective chemical recycling of waste plastics. The combined use of hydrocarbon and water is a new technique to control the reactivity of hightemperature water.
Establishment of an activated peroxide system for low-temperature cotton bleaching using N-[4-(triethylammoniomethyl)benzoyl]butyrolactam chloride
Xu, Changhai,Hinks, David,Sun, Chang,Wei, Qufu
, p. 71 - 77 (2015)
Cotton bleaching is traditionally carried out in strongly alkaline solution of hydrogen peroxide (H2O2) at temperatures close to the boil. Such harsh processing conditions can result in extensive water and energy consumptions as well as severe chemical damage to textiles. In this study, an activated peroxide system was established for low-temperature cotton bleaching by incorporating a bleach activator, namely N-[4-(triethylammoniomethyl)benzoyl]butyrolactam chloride (TBBC) into an aqueous H2O2 solution. Experimental results showed that the TBBC-activated peroxide system exhibited the most effective bleaching performance in a pH range of 6-8 which could be approximated by adding sodium bicarbonate (NaHCO3). The TBBC/H2O2/NaHCO3 system led to rapid bleaching of cotton at a temperature as low as 50°C. In comparison with the hot alkaline peroxide bleaching system, the TBBC/H2O2/NaHCO3 system provided cotton fabric with an equivalent degree of whiteness, higher degree of polymerization, and slightly lower water absorbency. The new activated peroxide system may provide a more environmentally benign approach to cotton bleaching.
An alternative efficient method for transformation of thiocarbonyl to carbonyl group using trifluoroacetic anhydride
Masuda, Ryoichi,Hojo, Masaru,Ichi, Tadaaki,Sasano, Shigetoshi,Kobayashi, Tatsuya,Kuroda, Chihiro
, p. 1195 - 1198 (1991)
A simple and efficient procedure for the rapid and mild conversion of thiocarbonyls to carbonyls in high yields is described.
Light-enhanced acid catalysis over a metal-organic framework
Xu, Caiyun,Sun, Keju,Zhou, Yu-Xiao,Ma, Xiao,Jiang, Hai-Long
, p. 2498 - 2501 (2018)
A Br?nsted acid-functionalized metal-organic framework (MOF), MIL-101-SO3H, was prepared for acid-engaged esterification reactions. Strikingly, for the first time, the MOF exhibits significantly light-enhanced activity and possesses excellent activity and recyclability, with even higher activity than H2SO4 under light irradiation.
Dehydration of 5-amino-1-pentanol over rare earth oxides
Ohta, Kaishu,Yamada, Yasuhiro,Sato, Satoshi
, p. 73 - 80 (2016)
Vapor-phase catalytic dehydration of 5-amino-1-pentanol was investigated over various oxide catalysts including rare earth oxides (REOs). Over ordinary acidic oxides such as Al2O3, SiO2, SiO2-Al2O3, TiO2, and ZrO2, a cyclic amine such as piperidine was mainly produced at temperatures of 300 °C and higher. In contrast, basic REOs with a cubic bixbyite structure showed the catalytic activity in the conversion of 5-amino-1-pentanol to produce 4-penten-1-amine at 425 °C. In REO catalysts, Tm2O3, Yb2O3, and Lu2O3 showed the high conversion of 5-amino-1-pentanol and the high selectivity to 4-penten-1-amine. Especially, Yb2O3 calcined at 800 °C showed a high formation rate of 4-penten-1-amine with the selectivity of ca. 90% at 425 °C. In comparing the reactivity of several amino alcohols to form the corresponding unsaturated amines, Yb2O3 effectively catalyzed the dehydration of 6-amino-1-hexanol into 5-hexen-1-amine, whereas 3-amino-1-propanol and 4-amino-1-butanol were not effectively dehydrated due to the decomposition of the reactant.
Thermal alkylation of ambidentate lactams with 2-(perfluoroalkyl)-1-iodoalkanes. The effect of reaction conditions and ring size on the synthesis of 2-(perfluoroalkyl)ethanols and the mechanism of reaction
Brace
, p. 2059 - 2071 (1995)
The perfluoroalkylated long chain alcohols and their derivatives exhibit strong surface activity in solution and novel surface modification properties as adsorbed layers or films. A new, little known synthesis of 2-(perfluoroalkyl)ethanols (R(F)CH2CH2OH) employs a lactam, e.g., 2-pyrrolidinone (2), with an iodoalkane, e.g., C6F13CH2CH2I (1). Alkylation of 2 by heating with 1 gives C6F13CH2CH2OH (3) in 83% distilled yield, and treating the residual lactim ether salt (6·HI) with K2CO3 gives additional 3, up to 94% yield. Rate of alcohol formation (k(alc)) is unaffected by molar ratio of 2:1, yet rate of 1 reaction (k(iodo)) increases 2.44 times with doubling of 2:1 and side product C6F13CH = CH2 (4) decreases from 4 to 2%. For homologous lactams [(CH2)(n)NHC = O] (n = 3-5), selectivities (mol 3:4) are as follows: 5-membered ring, 18.4; 6-membered ring, 0.73; 7-membered ring, 0.13. Conversions to 3 are as follows: 6-membered ring, 19.4%; and 7-membered ring, 1.75%. A three-stop mechanism is proposed: (1) O-alkylation of the lactam by 1 gives lactim salt I; (2) N-substitution of salt I by another molecule of lactam forms a tetrahedral adduct (II); (3) breakdown of salt II gives 3 and iminolactam salt III. In model experiments, heating of 2 and lactim 6 yields 99% of 3 and iminolactam 5 and 1% of 4. By contrast, 7-membered 14 with 2 gives 45% of 3 and iminolactam 12, besides 4 and -caprolactam 16 (20%). For higher lactams, two competitive reactions can be discerned: (1) the S(N)2 displacement of alcohol by N-attack on salt II and a unimolecular, concerted fragmentation of the lactim, to lactam and alkene.
N-ACETYL-ε-AMINOCAPROIC ACID. IV. HYDROLYSIS OF N-ACETYL-ε-CAPROLACTAM
Yasnitskii, B. G.,Dol'berg, E. B.,Spivak, A. L.
, p. 60 - 63 (1980)
-
-
Fukui,K. et al.
, p. 3168 - 3173 (1973)
-
VAPOR-PHASE BECKMANN REARRANGEMENT OF CYCLOHEXANONE OXIME OVER BORIA-HYDROXYAPATITE CATALYST
Izumi, Yusuke,Sato, Satoshi,Urabe, Kazuo
, p. 1649 - 1652 (1983)
The boron trioxide coupled with hydroxyapatite was found to catalyze the Beckmann rearrangement of cyclohexanone oxime in the vapor phase at 300 deg C more selectively than boria-alumina and silica-alumina.The basic property of hydroxyapatite appears to play an important role in effecting the rearrangement selectively.
Mechanistic insight into self-propagation of organo-mediated beckmann rearrangement: A combined experimental and computational study
An, Na,Tian, Bo-Xue,Pi, Hong-Jun,Eriksson, Leif A.,Deng, Wei-Ping
, p. 4297 - 4302 (2013)
Organo-mediated Beckmann rearrangement in the liquid phase, which has the advantage of high efficiency and straightforward experimental procedures, plays an important role in the synthesis of amides from oximes. However, the catalytic mechanisms of these organic-based promoters are still not well understood. In this work, we report a combined experimental and computational study on the mechanism of Beckmann rearrangement mediated by organic-based promoters, using TsCl as an example. A novel self-propagating cycle is proposed, and key intermediates of this self-propagating cycle are confirmed by both experiments and DFT calculations. In addition, the reason why cyclohexanone oxime is not a good substrate of the organo-mediated Beckmann rearrangement is discussed, and a strategy for improving the yield is proposed.
Strong basic sites accelerate the deactivation of oxide catalysts supported on FSM-16 for the vapor-phase beckmann rearrangement of cyclohexanone oxime
Shouro, Daisuke,Nakajima, Tsuyoshi,Mishima, Shozi
, p. 1319 - 1320 (1999)
The acidic and basic properties of FSM-16-supported Al2O3, ZnO or CdO were determined by the temperature-programmed desorption (TPD) of ethylamine. Relationship of the deactivation rate of the catalyst during the vapor-phase Beckmann rearrangement with the acidic and basic properties was studied. It was found that the deactivation was accelerated with an increase in the amount of the strong basic sites.
-
Sakai,I. et al.
, p. 3381 - 3383 (1979)
-
-
Just,Cunningham
, p. 1151 (1972)
-
Novel mesoporous silicoaluminophosphates as highly active and selective materials in the Beckmann rearrangement of cyclohexanone and cyclododecanone oximes
Conesa,Mokaya,Yang,Luque,Campelo,Romero
, p. 1 - 10 (2007)
Novel mesoporous silicoaluminophosphate molecular sieves with an MCM-41 type structure were synthesized and characterized using various techniques, including XRD, N2 adsorption, DRIFT, TEM, and 27Al, 29Si, and 31P MAS NMR. The surface acidity of the materials was determined by using pyridine and 2,6-dimethylpyridine as probe molecules. The catalytic activity of the mesoporous silicoaluminophosphates in the Beckmann rearrangement of cyclohexanone and cyclododecanone oximes was investigated and compared under gas- and liquid-phase (conventional vs microwave-assisted heating) conditions. The mesoporous silicoaluminophosphate materials are extremely active and selective in both reactions, giving yields comparable to those obtained on aluminosilicates and zeolites in the case of the vapor-phase Beckmann rearrangement of cyclohexanone oxime and the microwave-assisted Beckmann rearrangement of cyclododecanone oxime, respectively. The materials were also highly reusable after reactivation in vapor-phase reactions, with an increase in selectivity to the oxime, although a decrease in the durability (expressed as lifetime at quantitative conversion of the oxime) was also found.
-
Collum,D.B. et al.
, p. 4393 - 4394 (1978)
-
Thermolysis Reaction of 2-Acetyl-1-Oxo-Five-, Six-, and Seven-Membered Ring
Al-Awadi, Nouria A.,Mathew, Tommy
, p. 843 - 848 (1995)
The rates of gas-phase thermolysis reactions of 2-acetylcyclopentanone 1, 2-acetylcyclohexanone 2, N-acetylcaprolactam 3, 2-acetylbutyrolactone 4, 2-acetyl-2-methylbutyrolactone 5, and 3-acetyl-2-oxazolidinone 6 have been measured over a temperature range of 50 K.They undergo unimolecular first-order elimination reactions for which log A = 11.7, 11.7, 11.2, 11.4, 11.5, and 11.1 s-1 and Ea = 193.4, 189.5, 153.2, 201.0, 206.8, and 176.1 kJ mol-1, respectively.The effect of the ring size together with the effect of a heteroatom in the ring on the rate of thermolysis reactions for compounds 1-6 is the subject of this work.
-
Tada,Tokura
, p. 387 (1958)
-
High temperature calcination for a highly efficient and regenerable B2O3/ZrO2 catalyst for the synthesis of ε-caprolactam
Xu, Bo-Qing,Cheng, Shi-Biao,Zhang, Xin,Ying, Shuang-Feng,Zhu, Qi-Ming
, p. 1121 - 1122 (2000)
High temperature calcination of boria-supported zirconia leads to highly selective and regenerable B2O3/ZrO2 catalysts for the synthesis of ε- caprolactam by Beckmann rearrangement of cyclohexanone oxime.
Beckmann rearrangement of cyclohexanone oxime to ε-caprolactam in a modified catalytic system of trifluoroacetic Acid
Zhang,Riaud,Wang,Lu,Luo
, p. 151 - 157 (2014)
A catalytic system, including trifluoroacetic acid and organic solvent additives, was applied to carry out the Beckmann rearrangement of cyclohexanone oxime to ε-caprolactam. High conversion (100%) and high selectivity to caprolactam (>99%) have been successfully obtained using acetonitrile as the additive. The effect of several organic solvents on the reaction was investigated, and the catalyst composition was optimized. The results indicate that the catalytic system with 10 wt% of acetonitrile can give the fastest reaction rate. An immiscible twophase system was proposed to study the side reaction of oxime hydrolysis which determines the selectivity. Based on the results, a simplified reaction process was suggested and a mathematical kinetic model was developed. The performance of the catalytic system is much better than the classic process. Neutralization agent and ammonium sulfate by-product are both completely avoided.
Amide (A)–thioamide (T) interconversions using Ph3SiSH (A to T) and Ph3SnOH (T to A) reagents
Arias-Ugarte, Renzo N,Sharma, Hemant K,Pannell, Keith H
, p. 510 - 513 (2016)
Ph3SiSH transforms amides to thioamides and Ph3SnOH performs the reverse process, with the concomitant formation of Ph3SiOH (or Ph3SiOSiPh3) and Ph3SnSSnPh3, respectively. The chemistry is a delightful illustration of the oxophilicity of silicon compared to the thiophilicity of tin and occurs under relatively mild conditions, and for amide to thioamide transformation requires no amide activation. The chemistry is in accord with available data for Si?(S)(O), Sn?(O)(S) and C?(O)(S) bond energies. Copyright
-
Ohno,Sakai
, p. 4541 (1965)
-
Dehydrogenative Oxidation of Cyclic Amines on a Diruthenium Complex
Shimogawa, Ryuichi,Fujita, Ryosuke,Takao, Toshiro,Suzuki, Hiroharu
, p. 1893 - 1896 (2017)
The dehydrogenative oxidation of cyclic amines catalyzed by a diruthenium complex and mechanistic studies are described. Cyclic amines and water reacted in the presence of Cp?Ru(μ-H)4RuCp? (1) (Cp? = 1,2,4-tri-tert-butylcyclopentadienyl) to afford lactams accompanied by elimination of the hydrogen gas. The reaction of hexamethylenimine with 1 at 160 °C afforded Cp?Ru(μ-H)2(μ-C6H11N)RuCp? (2), having a novel μ-cyclic imine ligand through N-H and C-H bond cleavages. Further C-H bond cleavage of 2 proceeded at 180 °C to afford Cp?Ru(μ-H)(μ-η2:η2-C6H10N)RuCp? (4), having a perpendicularly coordinated imidoyl ligand. Complex 4 readily reacted with water and liberated -caprolactam. The cooperative interaction of the two ruthenium atoms leading to N-H and double C-H bond cleavages was the key to the dehydrogenative oxidation of cyclic amines.
Beckmann rearrangement of cyclohexanone oxime over borate-pillared LDHs
Lin, Jenn-Tsuen
, p. 779 - 787 (1999)
Borate-pillared layered double hydroxides (LDHs) were studied as catalysts in vapor-phase Beckmann rearrangement of cyclohexanone oxime to caprolactam under atmospheric pressure. The results were compared with those over the physical admixture of LDH and boria, the co-precipitate of B-Mg-Al hydroxide, and the pristine LDH and boria compounds. Although oxime conversion and caprolactam selectivity declined with time-on-stream over all the catalysts, borate-pillared LDH catalysts could retain activity longer than pure boria or those prepared by other methods. The decay rate however was affected by the Mg/Al ratio, the boron content and the form of LDH precursor used. The LDH itself functioning as a basic catalyst contributed to the formation of side products such as cyclohexanone and 2-cyclohexen-1-one. The physical admixtures of LDH and boria had catalytic properties more close to that of pure boria, which gave high caprolactam selectivities but lost the activity in several hours on stream. The high resistance to decay of the pillared catalysts was attributed to that boria in the interlayer of LDH was stabilized and prevented from structural transformation to a glassy state of no activity.
-
Turbak
, (1968)
-
Selective production of ε-caprolactam via liquid-phase Beckmann rearrangement of cyclohexanone oxime over HUSY catalyst
Ngamcharussrivichai, Chawalit,Wu, Peng,Tatsumi, Takashi
, p. 1288 - 1289 (2004)
ε-Caprolactam was selectively produced through the liquid-phase Beckmann rearrangement of cyclohexanone oxime over HUSY catalyst when the by-products formation was effectively retarded by blockage of the corresponding active centers of pre-adsorbed water and benzonitrile molecules.
-
Grakauskas,V. et al.
, p. 3839 - 3841 (1968)
-
Vapor-phase Beckmann rearrangement of cyclohexanone oxime over halide cluster catalysts
Nagashima, Sayoko,Kamiguchi, Satoshi,Ohguchi, Satoshi,Chihara, Teiji
, p. 135 - 138 (2011)
When a silica gel-supported tungsten halide cluster with an octahedral metal framework, (H3O)2[(W6Cl 8)Cl6]·6H2O/SiO2, is treated in a helium stream in the temperature range 250-350 °C, catalytic activity for the Beckmann rearrangement of cyclohexanone oxime develops. Niobium and tantalum clusters with the same metal framework also catalyze the reaction. Cyclopentanone oxime and acetone oxime also undergo Beckmann rearrangements over the tungsten cluster. The weak Br?nsted acidity (H0 ≈ +1.5) of the hydroxo ligand, which is developed on the activated cluster, is favorable for the rearrangement.
Enzymatic formation of lactams in organic solvents
Gutman, Arie L.,Meyer, Elazar,Yue, Xu,Abell, Chris
, p. 3943 - 3946 (1992)
Porcine pancreatic lipase in organic solvents catalyses the intramolecular cyclisation of aminoesters and the formation of macrocyclic bislactams from diesters and diamines.
Vapor phase Beckmann rearrangement using high silica zeolite catalyst
Forni,Fornasari,Giordano,Lucarelli,Katovic,Trifiro,Perri,Nagy
, p. 1842 - 1847 (2004)
Vapor phase Beckmann rearrangement of cyclohexanone oxime to ε-caprolactam has been studied using high silica zeolite catalysts. Catalysts with different crystal sizes and gel-ageing times have been activated by ionic exchange in different conditions by means of a highly basic solution and a nearly neutral solution both containing ammonium salts. Samples have been calcined at different temperatures in order modify the number of defective sites. We observed that samples exchanged by means of a highly basic solution (pH > 10)1.2 and calcined at a relatively lower temperature (450°C) show the most interesting catalytic results. X-ray powder diffraction patterns of these samples show2 retention of the unit cell symmetry (orthorhombic cell) if compared to the dried sample. NH 3-TPD confirms the low acidity of high silica zeolites, however a higher amount of desorbed ammonia is observed for the samples exchanged at higher pH and calcined at 450°C. Due to silanol nests the IR spectra of the same samples show the formation of Si-NH2 bonds which are absent in the same material exchanged by other methods. Such sites seem to promote the high stability of the high silica zeolite catalysts also to the regeneration which is needed to remove the heavy carbonaceous compounds from the catalyst surface.
A new procedure to obtain ?-caprolactam catalyzed by a guanidinium salt
Fernández-Stefanuto,Verdía,Tojo
, p. 12830 - 12834 (2017)
A new procedure to prepare ?-caprolactam by the Beckmann rearrangement of cyclohexanone oxime is described. Treatment of the oxime with the novel salt cyanoguanidinium tosylate affords ?-caprolactam without the need of any other promoter.
Gas-phase catalytic Beckmann rearrangement over crystalline BPO4 of dehydration ability
Tsuji, Hideto,Setoyama, Tohru
, p. 1232 - 1233 (2005)
The crystalline BPO4 with a P/B ratio around 1.5 prepared by dehydration of boric and phosphoric acid was found to be an effective heterogeneous catalyst for the gas-phase Beckmann rearrangement of cyclohexanone oxime to ε-caprolactam. Copyright
PREPARATION METHOD OF CAPROLACTAM
-
, (2022/03/14)
The present disclosure discloses a method for preparing caprolactam including: (1) contacting cyclohexanone oxime with a catalyst to carry out reaction in the presence of ethanol and under the condition of gas phase Beckmann rearrangement reaction of cyclohexanone oxime; (2) separating the reaction product obtained in step (1) to produce an ethanol solution of crude caprolactam, and then separating the ethanol solution of crude caprolactam to obtain ethanol and crude caprolactam; (3) removing impurities with boiling points lower than that of caprolactam in the crude caprolactam to obtain a light component removal product; (4) mixing the light component removal product with a crystallization solvent to carry out crystallization and solid-liquid separation to obtain a crystalline crystal; (5) subjecting the crystalline crystal to a hydrogenation reaction; wherein the crystallization solvent contains 0.1-2 wt % of ethanol.
Tandem Synthesis of ?-Caprolactam from Cyclohexanone by an Acidified Metal-organic Framework
Chen, Jingwen,Chen, Minda,Cruz, Andrew,Huang, Wenyu,Li, Xinle,Liu, Tianqing,Pei, Yuchen,Wu, Xun,Zhang, Biying
, p. 3084 - 3089 (2021/07/02)
Tandem synthesis of ?-caprolactam, one of the largest scaled commercial chemicals, is highly desired from the viewpoint of cost, energy, and environment. However, relevant studies have remained largely underexplored. By using a one-pot strategy, we encapsulated phosphotungstic acid (PTA) into a chromium terephthalate metal-organic framework (MOF), MIL-101, for the efficient tandem conversion of cyclohexanone to ?-caprolactam. The highly dispersed PTA in the MOF matrix showed a high yield of ?-caprolactam through a tandem oximation-Beckmann rearrangement reaction at 100 °C for 12 h. Moreover, MIL-101-PTA was recycled three times, with only a slight loss in their catalytic performance. To the best of our knowledge, this represents the first report using acidified MOF for a tandem oximation-Beckmann rearrangement reaction.
Efficient nitriding reagent and application thereof
-
Paragraph 0533-0535, (2021/03/31)
The invention discloses an efficient nitriding reagent and application thereof, wherein the nitriding reagent comprises nitrogen oxide, an active agent, a reducing agent and an organic solvent. By applying the nitriding reagent, nitrogen-containing compounds such as amide, nitrile and the like can be produced, and the method is simple in condition, low in waste discharge amount and simple in reaction equipment.