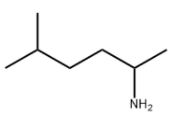
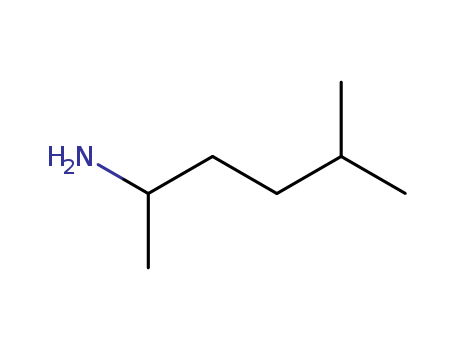2-AMINO-5-METHYLHEXANE literature
Biochemical and Structural Characterization of an (R)-Selective Transaminase in the Asymmetric Synthesis of Chiral Hydroxy Amines
Li, Fulong,Liang, Youxiang,Wei, Yuwen,Zheng, Yukun,Du, Yan,Yu, Huimin
, p. 4582 - 4589 (2021/08/07)
An (R)-selective transaminase RbTA with excellent stereoselectivity (>99% ee) in the asymmetric amination of hydroxy ketones was identified. Biochemical characterization showed that RbTA exhibited the highest activity toward 4-hydroxy-2-butanone among reported enzymes, and that it has broad substrate specificity, including for aliphatic, aromatic, and alicyclic ketones. Crystallization of RbTA were performed, as were molecular docking and mutagenesis studies. Residue Tyr125 plays a key role in substrate recognition by forming a hydrogen bond with hydroxy ketone. The applicability of the enzyme was determined in preparative-scale synthesis of (R)-3-amino-1-butanol, demonstrating the potential of RbTA as a green biocatalyst for production of value-added chiral hydroxy amines. This study provides an efficient tool for enzymatic synthesis of chiral hydroxy amines, as well as structural insight into substrate recognition by transaminases in the asymmetric amination of hydroxy ketones. (Figure presented.).
Importance of the lipophilic group in carbamates having histamine H3- receptor antagonist activity
Kiec-Kononowicz,Wiecek,Sasse,Ligneau,Elz,Ganellin,Schwartz,Stark,Schunack
, p. 349 - 355 (2007/10/03)
In order to evaluate changes in the lipophilic part of designed carbamates concerning their potential histamine H3-receptor antagonist properties a new series of O-[3-(1H-imidazol-4-yl)propanol]carbamates was derived containing N-mono- or dialkenyl, alkynyl, cycloalkyl, or double- branched alkyl substituents. The compounds were tested in vitro for their H3-receptor antagonist activity on synaptosomes of rat cerebral cortex and shared moderate to high antagonist activity in vitro. In this series 3-(1H- imidazol-4-yl)propyl N-(4-pentenyl)carbamate (4) was the most potent compound in vitro (K(i) = 6.3 nM). H3-receptor antagonist activity in the central nervous system (CNS) was detected for most compounds in the in vivo H3- receptor assay based upon measurement of brain N(τ)-methylhistamine levels after p.o. administration to mice. The most effective carbamate in vivo, 3- (1H-imidazol-4-yl)propyl N-(allyl)carbamate (3), showed higher CNS potency (ED50 = 0.48 mg/kg p.o.) than the reference antagonist thioperamide. For some novel carbamates their histamine H1- and H2-receptor activities were determined on isolated organs of guinea-pig thereby demonstrating their high H3-receptor selectivity.