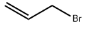
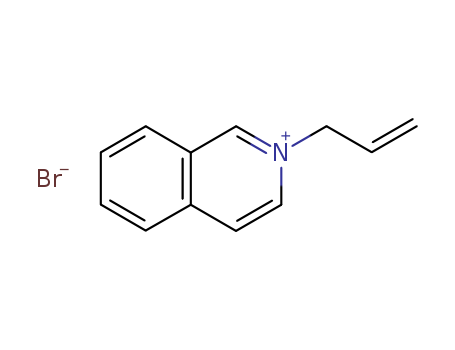
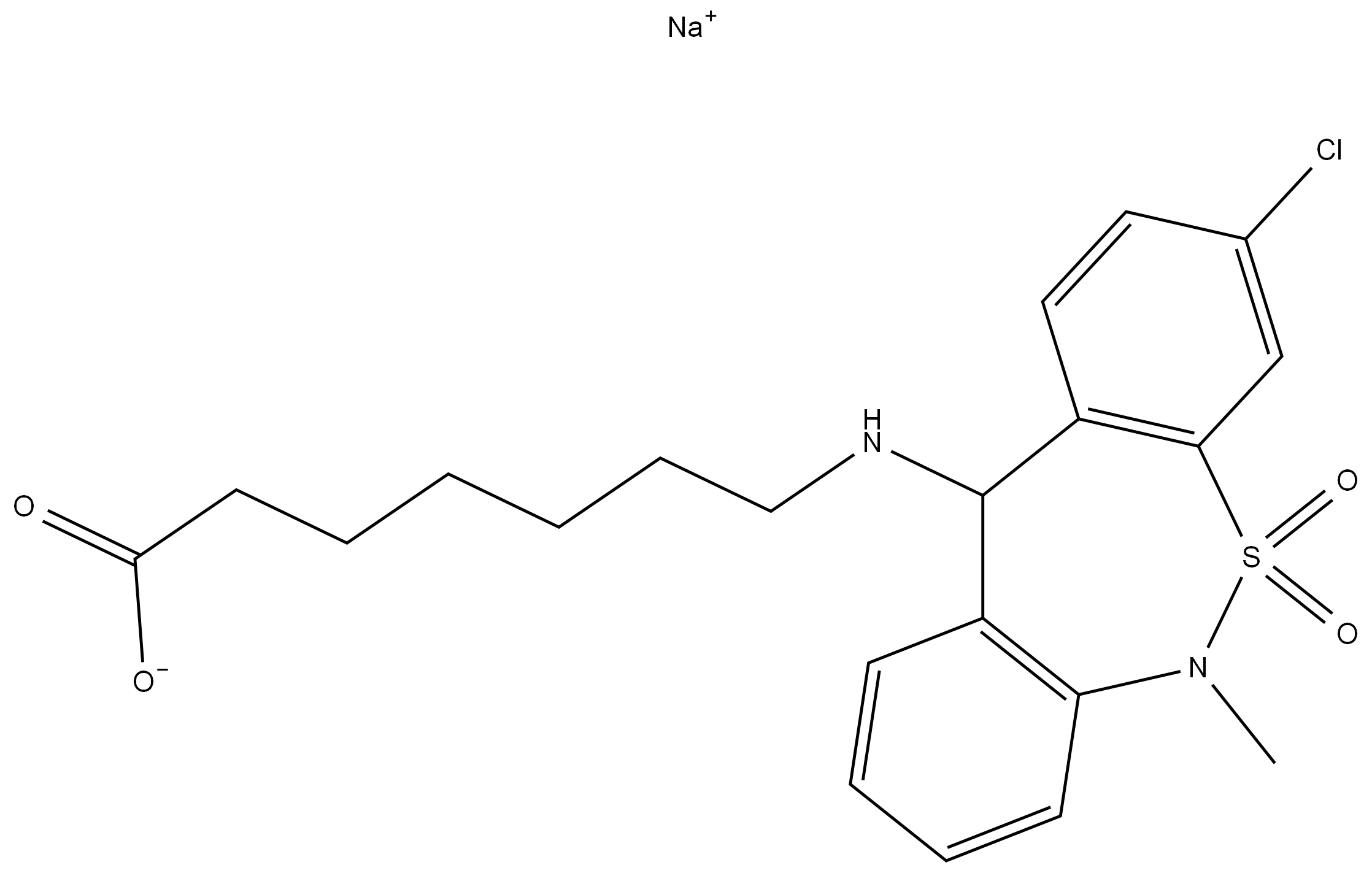
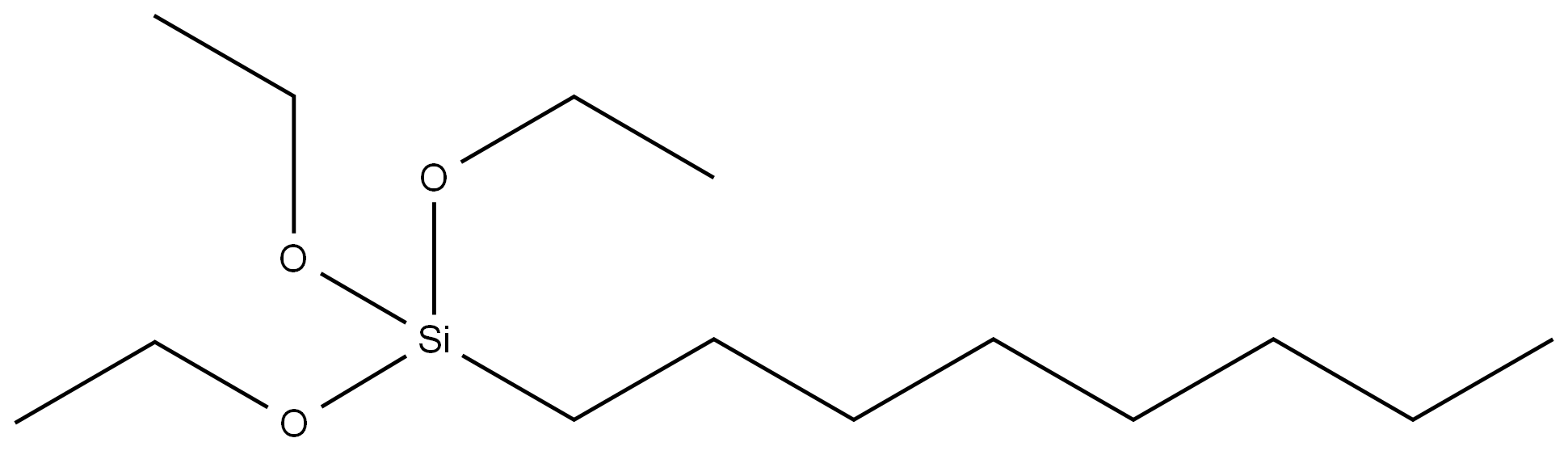
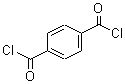
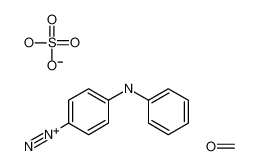
Allyl bromide literature
Kinetics of free radicals produced by infrared multiphoton-induced decompositions. 1. Reactions of allyl radicals with nitrogen dioxide and bromine
Slagle, Irene R.,Yamada, Fumiaki,Gutman, David
, p. 149 - 153 (1981)
A new versatile technique to study quantitatively the gaseous reactions of polyatomic free radicals is described in detail. Free radicals are generated homogeneously in a tubular reactor by the infrared multiphoton-induced decomposition (MPD) of suitable radical precursors. The concentrations of reactants and products (both stable and labile) are monitored by using photoionization mass spectrometry. Reactions of the allyl radical, generated by the MPD of allyl bromide, with nitrogen dioxide and bromine have been studied at 300 K. The measured rate constants are 3.9(±0.8) × 10-11 cm3 s-1 for the C3H5 + NO2 reaction and 9.0(±1.8) × 10-12 cm3 s-1 for the C3H5 + Br2 reaction. The potential of the experimental facility for other kinds of studies is discussed.
-
Anderson,Freenor
, p. 5037 (1964)
-
-
Frazer,Gerrard
, p. 3624,3626, 3627 (1955)
-
Synthesis of C-4-Allyloxy-3 Methoxyphenylcalix[4] resorcinarene from Vanillin and Its application as adsorbent of Pb(II) metal cation
Kesuma, Endhy Putra,Ohto, Keisuke,Siswanta, Dwi
, p. 769 - 775 (2016)
C-4-allyloxy-3-methoxyphenylcalix[4]resorcinarene had been synthesized from vanillin and its application as adsorbent for Pb(II) metal ion had been carried out. The synthesis was conducted in three steps to obtain the product as pink solid in 78.17% yield. The structure elucidation of the product was performed by IR,1H-NMR and 13C-NMR. Adsorption experiments were carried out in a batch system under variation of medium acidity, interaction time and initial metal concentration. Adsorption kinetics was studied using Lagergren and Ho model, while adsorption isotherm was analyzed by Langmuir and Freundlich equations. The result showed that the Pb(II) adsorption was optimum at pH 4 and at interaction time of 30 minutes. The kinetic study showed that the adsorption of Pb(II) followed pseudo-second order of Ho model with adsorption rate (k) of 1.176 g mg-1 minute-1 . The adsorption followed the Langmuir isotherm model with equilibrium constant (K) was 7.28?10-5 L mol-1 , adsorption capacity (qm) was 1.538 mmol g-1 (318.67 mg/g) and adsorption energy was 33.67 kJ mol-1 .
Kinetics of the Reactions of Unsaturated Hydrocarbon Free Radicals (Vinyl, Propargyl, and Allyl) with Molecular Bromine
Timonen, R. S.,Seetula, J. A.,Gutman, D.
, p. 8217 - 8221 (1993)
The kinetics of the reactions of three unsaturated free radicals (vinyl, propargyl, and allyl) with molecular bromine have been studied by using a tubular reactor coupled to a photoionization mass spectrometer.The radicals were homogeneously generated by the pulsed photolysis of precursor molecules at 193 nm.The subsequent decays of the radical concentrations were monitored in time-resolved experiments as a function of Br2 concentration to obtain the rate constants of these Br-atom metathesis reactions.Rate constants were measured as a function of temperature to obtain Arrhenius parameters.The following rate constant expressions were obtained (units of the preexponential factor are cm3 molecule-1 s-1 and activation energies are kJ mol-1; the temperature range covered in each study is also indicated): C2H3 + Br2 <(4.0 +/- 0.7)E-11 exp((2.4 +/- 1.5)/RT), 297-532 K>, C3H3 + Br2 <(2.8 +/- 0.5)E-12 exp((-2.3 +/- 1.2)/RT), 296-532 K>, and C3H5 +/- Br2 <(4.8 +/- 0.8)E-12 exp((1.6 +/- 0.8)/RT), 298-532 K>.The kinetics of R + Br2 reactions is reviewed, and the factors governing the reactivity of polyatomic free radicals in R + Br2 reactions are discussed.
-
Anderson Jr.,Freenor
, p. 626 (1972)
-
Generalized route to metal nanoparticles with liquid behavior
Warren, Scott C.,Banholzer, Matthew J.,Slaughter, Liane S.,Giannelis, Emmanuel P.,DiSalvo, Francis J.,Wiesner, Ulrich B.
, p. 12074 - 12075 (2006)
We report the generalized synthesis of metal nanoparticles with liquid-like behavior. We introduce a thiol-containing ionic liquid, N,N-dioctyl-N-(3-mercaptopropyl)-N-methylammonium bromide, which serves as a ligand for platinum, gold, palladium, and rhodium nanoparticles. A rapid reduction using THF-soluble metal salts in the presence of the thiol generates nanoparticles with tunable sizes and size distributions. The as-synthesized nanoparticles are a solid and decompose before melting. Upon exchange of the halide anion for an amphiphilic sulfonate anion, however, the nanoparticles exhibit liquid-like properties at room temperature. The liquids have high metal loadings; for example, the 2.7 nm platinum nanoparticle liquid is 36% platinum by mass. Copyright
O-eugenol: A versatile molecule for production of polyfunctional alkenes via organometallic catalysis
Al-Ayed, Abdullah Sulaiman,Hamdi, Naceur,Peruzzini, Maurizio
, p. 960 - 964 (2016)
In this study, the synthesis and cross metathesis of o-eugenol (2-allyl-6-methoxy phenol) has been investigated. Synthesis was conducted through two stages of reaction. The first step in the synthetic procedure was to obtain the intermediate 1-but-3-enyl-2-methoxy benzene. Then the heating of the obtained intermediate will initiate will initiate a [3,3] sigmatropic rearrangement to give the o-eugenol with a good yield. The ruthenium-catalyzed cross-metathesis of o-eugenol derivatives with electron deficient olefins including methyl acrylate, acrylonitrile and acrylamides was also reported. In addition the polymerization of 1-allyl-2-(allyloxy)-3-methoxybenzene was possible by acyclic diene metathesis and this allowed to synthesize a polymer from a natural substrate. All the resulting structures were supported by the spectroscopic data.
Synthesis and characterization of ferroelectric liquid crystalline siloxanes containing 4-hydroxyphenyl(2S,3S)-2-chloro-3-methylvalerate
Lin, Chih-Hung
, p. 33 - 42 (2012)
New series of organosiloxane ferroelectric liquid crystalline materials have been synthesized, and their mesomorphic and physical properties have been characterized. These new series contain bis-siloxane or tris-siloxane unit attached to the flexible alkyl chain end of (2S,3S)-2-chloro-3-methylvalerate. The siloxane molecule induction is helpful to the chiral smectic C (S C) formation and chiral SC* stabilization, and it simultaneously causes the liquid crystal temperature range of chiral S C* to be broader. The siloxane member is helpful in reducing the smectic C (SC) transation shift temperature, and the molecule containing tris-siloxane units shows better effect than the bis-siloxane one. The synthesis and characterization of the new FLCs materials which exhibit SC* phase at room temperature and higher spontaneous polarization are presented.
-
Grant,Swinbourne
, p. 620 (1966)
-
-
Philippi
, p. 277 (1929)
-
Nitrogen-fixing of ultrasmall Pd-based bimetallic nanoclusters on carbon supports
Chen, Ping,Liang, Hai-Wei,Shen, Shan-Cheng,Wang, Lei,Xu, Shi-Long,Yin, Peng,Zhang, Le-Le
, p. 297 - 304 (2020)
Synthesis of supported Pd-based bimetallic catalysts is of great importance in the heterogeneous catalysis field owing to their optimal geometric and electronic effects. Downsizing active metals to ultrasmall nanocluster (<2 nm), which is mandatory for maximizing the metal atom utilization, however remains as formidable synthesis challenges. Here, we present a general synthetic approach to sub-2 nm Pd-based bimetallic nanoclusters on porous nitrogen-doped carbon supports, in which the strong chemical interaction between metal and nitrogen largely suppresses the metal aggregation during the H2-reduction at 400–500 °C. Through the nitrogen-fixing strategy, we prepare 9 sub-2 nm Pd-based bimetallic nanocluster catalysts by conventional impregnation process. The prepared supported bimetallic Pd-Pb nanocluster catalyst exhibit a high turnover frequency of 1092 h?1 for the semihydrogenation of phenylacetylene under a mild condition (30 °C, 5 bar H2), along with a high selectivity of >93% to styrene, demonstrating the alloying and small-size effects in the bimetallic nanocluster catalysts.
-
Asahara,T. et al.
, p. 1130 - 1133 (1971)
-
Enantioselective synthesis of ammonium cations
Walsh, Mark P.,Phelps, Joseph M.,Lennon, Marc E.,Yufit, Dmitry S.,Kitching, Matthew O.
, p. 70 - 76 (2021/09/06)
Control of molecular chirality is a fundamental challenge in organic synthesis. Whereas methods to construct carbon stereocentres enantioselectively are well established, routes to synthesize enriched heteroatomic stereocentres have garnered less attention1–5. Of those atoms commonly present in organic molecules, nitrogen is the most difficult to control stereochemically. Although a limited number of resolution processes have been demonstrated6–8, no general methodology exists to enantioselectively prepare a nitrogen stereocentre. Here we show that control of the chirality of ammonium cations is easily achieved through a supramolecular recognition process. By combining enantioselective ammonium recognition mediated by 1,1′-bi-2-naphthol scaffolds with conditions that allow the nitrogen stereocentre to racemize, chiral ammonium cations can be produced in excellent yields and selectivities. Mechanistic investigations demonstrate that, through a combination of solution and solid-phase recognition, a thermodynamically driven adductive crystallization process is responsible for the observed selectivity. Distinct from processes based on dynamic and kinetic resolution, which are under kinetic control, this allows for increased selectivity over time by a self-corrective process. The importance of nitrogen stereocentres can be revealed through a stereoselective supramolecular recognition, which is not possible with naturally occurring pseudoenantiomeric Cinchona alkaloids. With practical access to the enantiomeric forms of ammonium cations, this previously ignored stereocentre is now available to be explored.
Clean protocol for deoxygenation of epoxides to alkenes: Via catalytic hydrogenation using gold
Fiorio, Jhonatan L.,Rossi, Liane M.
, p. 312 - 318 (2021/01/29)
The epoxidation of olefin as a strategy to protect carbon-carbon double bonds is a well-known procedure in organic synthesis, however the reverse reaction, deprotection/deoxygenation of epoxides is much less developed, despite its potential utility for the synthesis of substituted olefins. Here, we disclose a clean protocol for the selective deprotection of epoxides, by combining commercially available organophosphorus ligands and gold nanoparticles (Au NP). Besides being successfully applied in the deoxygenation of epoxides, the discovered catalytic system also enables the selective reduction N-oxides and sulfoxides using molecular hydrogen as reductant. The Au NP catalyst combined with triethylphosphite P(OEt)3 is remarkably more reactive than solely Au NPs. The method is not only a complementary Au-catalyzed reductive reaction under mild conditions, but also an effective procedure for selective reductions of a wide range of valuable molecules that would be either synthetically inconvenient or even difficult to access by alternative synthetic protocols or by using classical transition metal catalysts. This journal is
Reaction Acceleration Promoted by Partial Solvation at the Gas/Solution Interface
Qiu, Lingqi,Wei, Zhenwei,Nie, Honggang,Cooks, R. Graham
, p. 1362 - 1365 (2021/09/14)
The kinetics of organic reactions of different types in microvolumes (droplets, thin films, and sealed tubes) show effects of gas/solution interfacial area, reaction molecularity and solvent polarity. Partial solvation at the gas/solution interface is a major contributor to the 104-fold reaction acceleration seen in bimolecular but not unimolecular reactions in microdroplets. Reaction acceleration can be used to manipulate selectivity by solvent choice.
Nickel-Catalyzed Asymmetric Reductive 1,2-Carboamination of Unactivated Alkenes
He, Jun,Xue, Yuhang,Han, Bo,Zhang, Chunzhu,Wang, You,Zhu, Shaolin
supporting information, p. 2328 - 2332 (2020/01/08)
Starting from diverse alkene-tethered aryl iodides and O-benzoyl-hydroxylamines, the enantioselective reductive cross-electrophilic 1,2-carboamination of unactivated alkenes was achieved using a chiral pyrox/nickel complex as the catalyst. This mild, modular, and practical protocol provides rapid access to a variety of β-chiral amines with an enantioenriched aryl-substituted quaternary carbon center in good yields and with excellent enantioselectivities. This process reveals a complementary regioselectivity when compared to Pd and Cu catalysis.