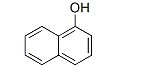
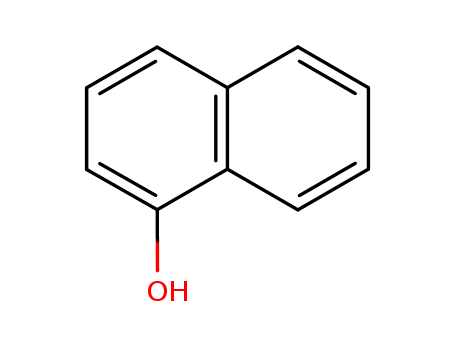
1-Naphthol literature
EXCEPTIONAL REACTIVITY OF THE AROMATIC RING IN 8-SUBSTITUTED 1-NAPHTHOL DERIVATIVES. READY REDUCTION TO TETRALINS.
Kirby, Anthony J.,Percy, Jonathan M.
, p. 6911 - 6920 (1988)
8-Lithio-1-methoxynaphthalene is acylated and alkylated to give 8-CO2H, COCH3 and Me2C(OH) derivatives in only poor yields.Methyl lithium does not add to the 8-acetyl compound, converting it instead to the lithium enolate.Compounds with secondary or tertiary alkyl peri-substituents undergo rapid protodealkylation, and are readily reduced to tetralins by H2/Pd at atmospheric pressure.
Dediazoniation of 1-naphthalenediazonium tetrafluoroborate in aqueous acid and in micellar solutions
Bravo-Diaz, Carlos,Pastoriza-Gallego, Maria Jose,Losada-Barreiro, Sonia,Sanchez-Paz, Veronica,Fernandez-Alonso, Alejandra
, p. 301 - 309 (2008)
We have measured the rates and product yields of dediazoniation of 1-naphthalenediazonium (1ND) tetrafluoroborate in the presence and absence of sodium dodecyl sulfate (SDS) micellar aggregates by employing a combination of UV-vis spectroscopy and high-performance liquid chromatography (HPLC) measurements. Kinetic data were obtained by a derivatization procedure with product yields were determined by HPLC. HPLC chromatograms show that in aqueous acid and in micellar solutions only one dediazoniation product is formed in significant quantities, 1-naphthol (NOH), and the observed rate constants (kobs) are tne same when IND loss is monitored spectrometrically and when NOH formation is monitored by HPLC. Activation parameters were obtained both in the presence and absence of SDS micellar aggregates. In both the systems, the enthalpies of activation are high and the entropies of activation are positive. The enthalpy of activation in the absence of SDS is very similar to that in the presence of SDS micelles, but the entropy of activation is lower by a factor of 4. As a consequence, SDS micelles speed up the thermal decomposition of 1 ND and increase kobs by a factor of 1.5 when [SDS] = 0.02 M. In contrast, results obtained in the presence of complexing systems such as crown ethers and polyethers show significant stabilization of the parent arenediazonium ions. Kinetic and HPLC data are consistent with the heterolytic DN + AN mechanism that involves the rate-determining fragmentation of the arenediazonium ion into a very reactive phenyl cation that reacts competitively with available nucleophiles.
-
Dusart,Bardy
, p. 1051 (1872)
-
Ultrasensitive self-enhanced electrochemiluminescence sensor based on novel PAN?Ru?PEI?Nafion nanofiber mat
Li, Libo,Liu, Xiaohong,You, Tianyan,Zhou, Limin
, p. 3590 - 3597 (2020)
In the present work, a novel self-enhanced electrochemiluminescence (ECL) composite was for the first time prepared by simply electrospinning a mixture of polyacrylonitrile (PAN), Ru(bpy)32+, poly(ethylenimine) (PEI) and Nafion, and defined as PAN?Ru?PEI?Nafion nanofiber mat (PAN?Ru?PEI?Nafionnfm). Herein, Ru(bpy)32+was applied as an ECL reagent, and PEI was selected as a co-reactant due to the abundant tertiary amine groups in its backbone. In order to further improve the ECL efficiency of the Ru(bpy)32+-PEI system, we innovatively loaded Ru(bpy)32+and PEI into PAN nanofibers simultaneously by a one-step electrospinning technique. As a result, the electronic transmission distance between them was sharply shortened. Meanwhile, with the help of electrostatic interaction between positively charged Ru(bpy)32+or PEI and negatively charged Nafion, exfoliation of Ru(bpy)32+and PEI from the PAN nanofiber mat was effectively avoided. As expected, the luminous nanofibers exhibited excellent ECL performance including high efficiency and good stability. On this basis, we fabricated a new self-enhanced ECL sensor with excellent analysis performance. Using a-naphthol as an analytical probe, the sensor showed a wide linear range from 1.0 × 10-12to 1.0 × 10-7M with a low detection limit of 1.0 × 10-12M. When it was applied to detect a-naphthol from carbaryl hydrolysis, the results were well consistent with those obtained from the high efficiency liquid chromatography-diode array detector (HPLC-DAD) method, indicating the potential application of the ECL sensor in real sample assays.
A validated stability indicating rapid LC method for duloxetine HCl
Srinivasulu,Srinivas,Reddy,Mukkanti,Buchireddy
, p. 10 - 13 (2009)
The present paper describes the development of a reversed phase liquid chromatographic (RPLC) analytical method for duloxetine HCl in the presence of its impurities and degradation products generated from forced decomposition studies. The drug substance w
Serum albumins and detoxication of anti-cholinesterase agents
Sogorb, Miguel A.,Vilanova, Eugenio
, p. 325 - 329 (2010)
Serum albumin displays an esterase activity that is capable of hydrolysing the anti-cholinesterase compounds carbaryl, paraoxon, chlorpyrifos-oxon, diazoxon and O-hexyl, O-2,5-dichlorphenyl phosphoramidate. The detoxication of all these anti-cholinesterase compounds takes place at significant rates with substrate concentrations in the same order of magnitude as expected during in vivo exposures, even when these substrate concentrations are between 15 and 1300 times lower than the recorded Km constants. Our data suggest that the efficacy of this detoxication system is based on the high concentration of albumin in plasma (and in the rest of the body), and not on the catalytic efficacy itself, which is low for albumin. We conclude the need for a structure-activity relationship study into the albumin-associated esterase activities because this protein is universally present in vertebrates and could compensate for reduced levels of other esterases, i.e., lipoprotein paraoxonase, in some species. It is also remarkable that the biotransformation of xenobiotics can be reliably studied in vitro, although conditions as similar as possible to in vivo situations are necessary.
Rabbit serum albumin hydrolyzes the carbamate carbaryl
Sogorb, Miguel A.,Carrera, Victoria,Benabent, Monica,Vilanova, Eugenio
, p. 520 - 526 (2002)
One of the main detoxification processes of the carbamate insecticides is the hydrolysis of the carbamic ester bond. Carboxylesterases seem to play important roles in the metabolization of carbamates. This study performs a biochemical characterization of the capabilities of rabbit serum albumin (RSA) to hydrolyze the carbamate carbaryl. Rabbit serum albumin was able to hydrolyze carbaryl with a Kcat of 7.1 × 10-5 s-1. The Km for this hydrolysis reaction was 240 μM. Human, chicken, and bovine serum albumins were also able to hydrolyze carbaryl. The divalent cation Cu2+ at 1 mM concentration inhibited around 50% of the hydrolysis of carbaryl by RSA. Other mono- and divalent cations at 1 mM concentration and 5 mM EDTA exerted no significant effects on the hydrolysis of carbaryl by RSA. The inhibition of the carbaryl hydrolysis by sulfydril blocking agents suggests that a cysteine residue plays an important role in the active center of the catalytic activity. Both caprylic and palmitic acids were noncompetitive inhibitors of the carbaryl hydrolysis by RSA. The carboxyl ester p-nitrophenyl butyrate is a substrate of RSA and competitively inhibited the hydrolysis of carbaryl by this protein, suggesting that the hydrolysis of carbaryl and the hydrolysis of carboxyl esters occur in the same catalytic site and through a similar mechanism. This mechanism might be based on the carbamylation of a tyrosine residue of the RSA. Serum albumin is a protein universally present in nontarget species of insecticides; therefore, the capability of this protein to hydrolyze other carbamates must be studied because it might have important toxicological and ecotoxicological implications.
Selective hydroxylation of naphthalene using the H2O2-dependent engineered P450BM3 driven by dual-functional small molecules
Chen, Zhifeng,Chen, Jie,Ma, Nana,Zhou, Haifeng,Cong, Zhiqi
, p. 831 - 836 (2018)
We herein report the H2O2-dependent selective hydroxylation of naphthalene catalyzed by engineered P450BM3 with the assistance of dual-functional small molecules (DFSMs). The mutation at position 268 significantly improved the hydroxylation activity of P450BM3, which is quite different from those engineered P450BM3 peroxygenases and NADPH-dependent P450BM3 mutants previously reported, implicating the unique role of the residue at position 268. This study provides a potential approach to develop the practical hydroxylation biocatalyst of P450s for aromatic hydrocarbons using the DFSM-facilitated P450BM3-H2O2 system.
Photocatalytic conversion of naphthalene to α-naphthol using nanometer-sized TiO2
Shi, Huixian,Zhang, Tianyong,Wang, Hongliang,Wang, Xiao,He, Meng
, p. 46 - 50 (2011)
The effects of various parameters (co-solvents, electron acceptors, and surface modification) on the direct synthesis of α-naphthol from naphthalene using photocatalytic processes were investigated. The OH radicals generated on UV-illuminated TiO2 photocatalysts led to the direct hydroxylation of naphthalene to α-naphthol. The addition of Fe 3+, Fe2+, Fe3+ + H2O2, and Fe2+ + H2O2 greatly increases the conversion of naphthalene and the yield of α-naphthol in TiO2 suspensions. The addition of Fe3+ + H2O2 to a TiO2 suspension increased the yield to 22.2. Surface modified-TiO2 had a significant influence on the hydroxylation reaction. La-Eu/TiO2, La-Y/TiO2, H3PW 12O40/TiO2, H3PMo12O 40/TiO2, Fe/TiO2, Ag/TiO2, Cu/TiO2, and N/TiO2 enhanced the conversion and yield more than TiO2. Fe/TiO2 has the highest photocatalytic efficiency among these species.
Palladium/zinc co-catalyzed syn-stereoselectively asymmetric ring-opening reaction of oxabenzonorbornadienes with phenols
Li, Sifeng,Xu, Jianbin,Fan, Baomin,Lu, Zhiwu,Zeng, Chaoyuan,Bian, Zhaoxiang,Zhou, Yongyun,Wang, Jun
, p. 9003 - 9007 (2015)
A new palladium/zinc co-catalyst system associated with chiral (R)-Difluorphos for asymmetric ring-opening reaction of oxabenzonorbornadienes with phenols is reported. This catalyst system allows the formation of cis-2-aryloxy-1,2-dihydronaphthalen-1-ol products in good yields (up to 95-% yield) with excellent enantioselectivities (up to 99-% ee). The cis-configuration of the product has been confirmed by X-ray crystal structure analysis. To the best of our knowledge, it represents the first example in ring-opening reactions of bicycloalkenes with heteronucleophiles in a syn-stereoselective manner. Open the ring: A new palladium/zinc co-catalyst associated with chiral (R)-Difluorphos for asymmetric ring-opening reaction of oxabenzonorbornadienes with phenolic or naphtholic nucleophiles was developed, which afforded the corresponding cis-2-aryloxy-1,2-dihydronaphthalen-1-ol products in good yields (up to 95-% yield) with excellent enantioselectivities (up to 99-% ee).
Determination of the hydrolysis kinetics of α-naphthyl acetate in micellar systems and the effect of HPMC (catalyst present)
Werawatganone, Pornpen,Wurster, Dale Eric
, p. 448 - 458 (2007)
The change in the hexadecyltrimethylammonium bromide (CTAB) critical aggregation concentration (CAC) was studied in the presence of various concentrations and grades of hydroxypropylmethyl cellulose (HPMC) using surface tension measurement (duNouey ring and Wilhelmy plate) and oil red O solubilization. According to the surface tension methods, the CAC was higher than the CTAB critical micelle concentration (CMC). CAC and CMC were not different when the solubilization method was used. Micellar solutions of CTAB have been found to accelerate the hydrolysis of α-naphthyl acetate (α-NA) by o-iodosobenzoic acid (IBA), a strong nucleophile. Pseudo-first-order kinetics were utilized for rate constant determination. The observed rate constants for the degradation of α-NA in the presence of varying CTAB concentrations with and without HPMC were analyzed according to the pseudophase model. The micellar rate constants and the micellar binding constants for the substrates were obtained. The presence of HPMC retarded the reaction rate, and the rate constant decreased as the polymer concentration increased. However, there was no obvious difference in the observed rate constants among the different grades of HPMC (Methocel E5, Methocel E15, Methocel E50). The decrease in the rate constant was likely due to the polymer-micelle interaction interfering with substrate binding to the CTAB micelles.
-
Hawthorne
, p. 1001 (1957)
-
Imidazolium-urea low transition temperature mixtures for the UHP-promoted oxidation of boron compounds
Martos, Mario,Pastor, Isidro M.
, (2022/01/03)
Different carboxy-functionalized imidazolium salts have been considered as components of low transition temperature mixtures (LTTMs) in combination with urea. Among them, a novel LTTM based on 1-(methoxycarbonyl)methyl-3-methylimidazolium chloride and urea has been prepared and characterized by differential scanning calorimetry throughout its entire composition range. This LTTM has been employed for the oxidation of boron reagents using urea-hydrogen peroxide adduct (UHP) as the oxidizer, thus avoiding the use of aqueous H2O2, which is dangerous to handle. This metal-free protocol affords the corresponding alcohols in good to quantitative yields in up to 5 mmol scale without the need of further purification. The broad composition range of the LTTM allows for the reaction to be carried out up to three consecutive times with a single imidazolium salt loading offering remarkable sustainability with an E-factor of 7.9, which can be reduced to 3.2 by the threefold reuse of the system.
Aryl phenol compound as well as synthesis method and application thereof
-
Paragraph 0173-0176, (2021/05/12)
The invention discloses a synthesis method of an aryl phenol compound shown as a formula (3). All systems are carried out in an air or nitrogen atmosphere, and visible light is utilized to excite a photosensitizer for catalyzation. In a reaction solvent, ArNR1R2 as shown in a formula (1) and water as shown in a formula (2) are used as reaction raw materials and react under the auxiliary action of acid to obtain the aryl phenol compound as shown in a formula (3). The ArNR1R2 in the formula (1) can be primary amine and tertiary amine, can also be steroid and amino acid derivatives, and can also be drugs or derivatives of propofol, paracetamol, ibuprofen, oxaprozin, indomethacin and the like. The synthesis method has the advantages of cheap and easily available raw materials, simple reaction operation, mild reaction conditions, high reaction yield and good compatibility of substrate functional groups. The fluid reaction not only can realize amplification of basic chemicals, but also can realize amplification of fine chemicals, such as synthesis of drugs propofol and paracetamol. The invention has wide application prospect and use value.
Me3SI-promoted chemoselective deacetylation: a general and mild protocol
Gurawa, Aakanksha,Kashyap, Sudhir,Kumar, Manoj
, p. 19310 - 19315 (2021/06/03)
A Me3SI-mediated simple and efficient protocol for the chemoselective deprotection of acetyl groups has been developedviaemploying KMnO4as an additive. This chemoselective deacetylation is amenable to a wide range of substrates, tolerating diverse and sensitive functional groups in carbohydrates, amino acids, natural products, heterocycles, and general scaffolds. The protocol is attractive because it uses an environmentally benign reagent system to perform quantitative and clean transformations under ambient conditions.
PREPARING METHODE FOR NAPHTHOL
-
Paragraph 0032-0048, (2021/01/28)
The present invention provides a method for producing naphthol by oxidizing naphthalene and an oxidizing agent in the presence of a catalyst. According to the present invention, naphthol can be produced in a single process of direct oxidation of naphthalene, and the yield and selectivity of naphthalene can be improved by controlling process variables such as the molar ratio of the oxidizing agent and naphthalene, catalyst input amount, and reaction time.