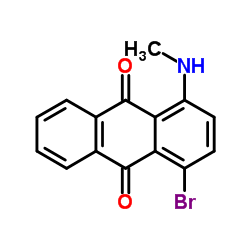
1-Methylamino-4-bromo anthraquinone literature
Picosecond Fluorescence Lifetimes of Anthraquinone Derivatives. Radiationless Deactivation via Intra- and Intermolecular Hydrogen Bonds
Inoue, Haruo,Hida, Mitsuhiko,Nakashima, Nobuaki,Yoshihara, Keitaro
, p. 3184 - 3188 (1982)
Radiationless deactivations from the S1(1CT) states of seven 1- and 2-substituted aminoanthraquinones were investigated by measuring picosecond fluorescence lifetimes and fluorescence quantum yields in benzene, acetonitrile, and ethanol.The S1(1CT) states of all derivatives were largely deactivated in ethanol.Deuterium isotope effects of the solvents on the nonradiative rate constants knr(in EtOH)/knr(in EtOD) = 9.0 (1-NH2 (2)), 2.1 (2-NH2 (5)), and 1.7 (2-piperidino (7)) were observed.The fluorescence quantum yield in ethanol was not affected by temperature (278-343 K).In benzene the excited 1-aminoanthraquinones were deacvated faster than 2-aminoanthraquinones.These observations were interpreted in terms of the radiationless deactivations od S1(1CT) through the intra- and intermolecular hydrogen bonds.
Acid-aided selectivity in bromination of 1-aminoanthraquinones
Chamberlin
, p. 27 - 31 (1995)
Selectivity in the bromination of 1-aminoanthraquinones was studied. The addition of mineral acids was surprisingly found to enhance selectivity for the four isomer and consequently give an enhanced yield.
Efficient, facile metal free protocols for the bromination of commercially important deactivated aminoanthracene-9,10-diones
Patil, Vilas V.,Gayakwad, Eknath M.,Patel, Khushbu P.,Shankarling, Ganapati S.
, p. 2608 - 2613 (2017)
Highly efficient, mild synthetic protocols were developed for the oxidative bromination of deactivated aminoanthracene-9,10-diones by using H2O2-HBr and m-CPBA-HBr in methanolic medium. Both the protocols offer excellent bromine atom economy, good conversion (100%) along with high yield (82–93%) and high purity of desired product. The N-alkylated amines undergo regio-selective bromination to give selective p-bromo product. The commercial availability of all the starting materials, simple reaction procedure and ease of work up, and easily amenable for scale up demonstrated commercial feasibility of both the protocols.
Synthesis and color properties of novel polymeric dyes based on grafting of anthraquinone derivatives onto O-carboxymethyl chitosan
Lv, Dongjun,Cui, Jin,Wang, Yufang,Zhu, Guohua,Zhang, Mingjie,Li, Xiujing
, p. 33494 - 33501 (2017/07/13)
Four polymeric dyes were prepared by grafting brominated anthraquinone derivatives onto O-carboxymethyl chitosan through Ullmann condensation. The chemical structure of the prepared polymeric dyes was determined by Fourier transform infrared spectroscopy (FT-IR), elemental analysis and differential scanning calorimetry (DSC), and the results showed that the anthraquinone derivatives were successfully grafted onto O-carboxymethyl chitosan. The grafting degrees of the four polymeric dyes were 0.66, 1.14, 0.93 and 1.01 mmol g-1 respectively. The color performance of brominated anthraquinone derivatives and prepared polymeric dyes was determined and compared using digital photographs and UV-Vis adsorption spectra, and it was found that the electronic properties and planarity of the substituent group in the anthraquinone derivatives obviously affected the adsorption wavelength of the prepared polymeric dyes. In addition, the polymeric dyes with an electron donating group and higher planarity showed longer adsorption wavelengths and more deep color. The cytotoxicity of prepared polymeric dyes was tested, and their IC50 values on human liver cell lines LO2 were 7.666, 8.557, 8.186 and 8.934 g L-1 respectively, suggesting low cytotoxicity of the prepared polymeric dyes.
Process for preparing substituted aminoanthraquinones
-
, (2008/06/13)
Substituted aminoanthraquinone compounds, which are used for dye stuffs or intermediate thereof, represented by the formula (II) STR1 wherein R3 represents a C1 -C6 alkyl group which may be substituted, X represents a hydrogen atom, --COR1 or --SO2 R2 wherein R1 and R2 each represents a substituted or unsubstituted C1 -C4 alkyl or C6 -C12 aryl group, and Y and Z represent independently a hydrogen atom, a halogen atom, a nitro group or a C1 -C4 alkyl group, is prepared by allowing anthraquinone compounds represented by the formula STR2 wherein X, Y and Z are as defined above, to react with alkylating agents in organic solvents in the presence of organic quaternary ammonium salts and alkalies.