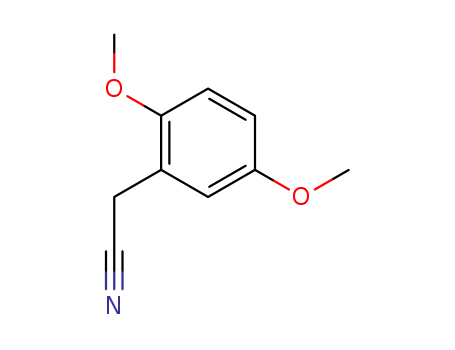
2,5-DIMETHOXYPHENYLACETONITRILE literature
Preparation method of 2,5-dimethoxyphenylacetic acid
-
Paragraph 0021; 0023; 0025, (2021/02/06)
The invention belongs to the technical field of drug synthesis, and particularly relates to a preparation method of 2,5-dimethoxyphenylacetic acid, wherein the preparation method comprises the following steps: A, reacting 1,4-dimethoxybenzene with formaldehyde under the action of hydrogen halide to obtain 2-halomethyl-1,4-dimethoxybenzene; B, reacting 2-halomethyl-1,4-dimethoxybenzene obtained inthe step A with a cyanation reagent, then diluting, washing, drying and concentrating under reduced pressure, and finally carrying out reduced pressure distillation to obtain 2(2,5-dimethoxyphenyl)acetonitrile; and C, hydrolyzing the 2(2,5-dimethoxyphenyl)acetonitrile obtained in the step B, cooling, extracting, merging, drying, concentrating under reduced pressure and purifying to finally obtainthe 2,5-dimethoxyphenylacetic acid. The yield and the total yield of the 2,5-dimethoxyphenylacetic acid obtained by the method disclosed by the invention are both higher than those of the 2,5-dimethoxyphenylacetic acid synthesized by a Willgerodt-Kindler method, and the yield and the total yield of the 2,5-dimethoxyphenylacetic acid are higher than those of 2,5-dimethoxyphenylacetic acid synthesized by the Willgerodt-Kindler method.
Controlled conversion of phenylacetic acids to phenylacetonitriles or benzonitriles using bis(2-methoxyethyl)aminosulfur trifluoride
Kangani, Cyrous O.,Day, Billy W.,Kelley, David E.
, p. 914 - 918 (2008/09/17)
A mild, efficient, and practical method for the one-step synthesis of benzonitriles from phenylacetic acids using bis(2-methoxyethyl)aminosulfur trifluoride is described. The reaction was easily extended to the synthesis of the corresponding phenylacetonitriles by inclusion of triethylphosphine.
Fluorinated biphenyls from aromatic arylations with pentafluorobenzenediazonium and related cations. Competition between arylation and azo coupling
Kosynkin, Dmitry,Bockman, T. Michael,Kochi, Jay K.
, p. 2003 - 2012 (2007/10/03)
High yields of the mixed perfluorinated biaryls (C6F5-Ar) are obtained by the catalytic dediazonlatlon of the pentafluorobenzenediazonium salt (C6F5N2+BF4-) in acetonitrile solutions containing various aromatic substrates (ArH) together with small amounts of iodide salts. Activated (electron-rich) as well as deactivated (electron-poor) arenes are successfully pentafluorophenylated by this method. The arylation is distinct from the azo coupling of the same substrates, which takes place in the absence of the iodide catalyst and yields the corresponding diazene (C6F5N=N-Ar) as product. The catalytic role of iodide, and the isomeric product distributions obtained with this procedure indicate that the arylation proceeds via the pentafluorophenyl radical in a efficient homolytic chain process. Since azo coupling involves electrophilic aromatic substitution of electron-rich ArH by C6F5N2+, the two competing pathways are distinct and do not have reactive intermediates in common.
Degradation of 3-aryl-2-hydroxyiminopropionic acids into arylacetonitriles using 1,1'-carbonyldiimidazole or 2,2'-oxalyldi(o-sulfobenzimide)
Kitagawa,Kawaguchi,Inoue,Katayama
, p. 3030 - 3033 (2007/10/02)
1,1'-Carbonyldiimidazole (1) is a useful reagent for the preparation of arylacetonitriles (9) from 3-aryl-2-hydroxyiminopropionic acids (8), and 2,2'-oxalyldi (o-sulfobenzimide) (2) can also be used for this purpose under essentially neutral conditions.