Unii-950o97nupo literature
Fluorinated adenosine A2A receptor antagonists inspired by preladenant as potential cancer immunotherapeutics
Yuan, Gengyang,Jankins, Tanner C.,Patrick, Christopher G.,Philbrook, Phaethon,Sears, Olivia,Hatfield, Stephen,Sitkovsky, Michail,Vasdev, Neil,Liang, Steven H.,Ondrechen, Mary Jo,Pollastri, Michael P.,Jones, Graham B.
, (2017)
Antagonism of the adenosine A2A receptor on T cells blocks the hypoxia-adenosinergic pathway to promote tumor rejection. Using an in vivo immunoassay based on the Concanavalin A mouse model, a series of A2A antagonists were studied and identified preladenant as a potent lead compound for development.Molecular modeling was employed to assist drug design and subsequent synthesis of analogs and those of tozadenant, including fluorinated polyethylene glycol PEGylated derivatives. The efficacy of the analogs was evaluated using two in vitro functional bioassays, and compound 29, a fluorinated triethylene glycol derivative of preladenant, was confirmed as a potential immunotherapeutic agent.
PROCESS AND INTERMEDIATES FOR THE PREPARATION OF PRELADENANT AND RELATED COMPOUNDS
-
, (2012/10/08)
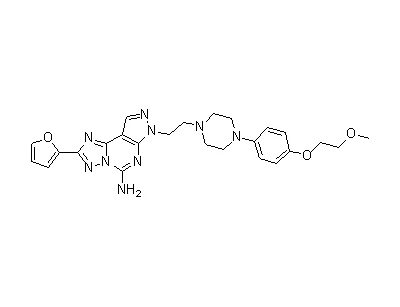
The present invention describes processes for the synthesis of 2-(furan-2-yl)-7-[2-[4-[4-(2-methoxyethoxy)phenyl]piperazin-l-yl] ethyl]-7H-pyrazolo[ 4,3-e ][1,2,4]- triazolo[1,5c] pyrimidin-5-amine (Preladenant) represented by the structure of formula (1
Potent, selective, and orally active adenosine A2A receptor antagonists: Arylpiperazine derivatives of pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidines
Neustadt, Bernard R.,Hao, Jinsong,Lindo, Neil,Greenlee, William J.,Stamford, Andrew W.,Tulshian, Deen,Ongini, Ennio,Hunter, John,Monopoli, Angela,Bertorelli, Rosalia,Foster, Carolyn,Arik, Leyla,Lachowicz, Jean,Ng, Kwokei,Feng, Kung-I
, p. 1376 - 1380 (2008/02/05)
Antagonism of the adenosine A2A receptor offers great promise in the treatment of Parkinson's disease. Employing the known pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine A2A antagonist SCH 58261 as a starting point, we identified the potent and selective (vs. A1) antagonist 11 h, orally active in the rat haloperidol-induced catalepsy model. We further optimized this lead to the methoxyethoxyethyl ether 12a (SCH 420814), which shows broad selectivity, good pharmacokinetic properties, and excellent in vivo activity.
PROCESS FOR PREPARING SUBSTITUTED 5-AMINO-PYRAZOLO-[4,3-E]-1,2,4-TRIAZOLO[1,5-C]PYRIMIDINES
-
Page/Page column 17, (2008/06/13)
A process for preparing substituted 5-amino-pyrazolo[4,3-e]-1,2,4-triazolo-[1,5-c]pyrimidine compounds having an aminoalkyl substituent at the 7-position is disclosed.