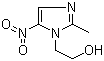
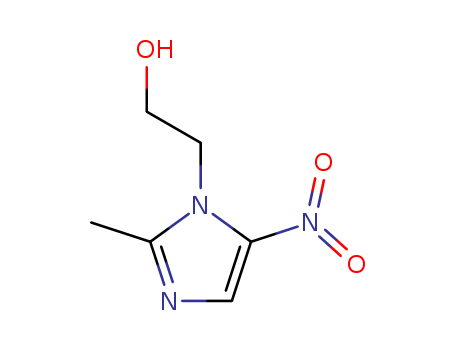
Metronidazole literature
SYNTHESIS OF METRONIDAZOLE FROM ETHYLENEDIAMINE
Kraft, M. Ya.,Kochergin, P. M.,Tsyganova, A. M.,Shlikhunova, V. S.
, p. 861 - 863 (1989)
-
-
Ayscough et al.
, (1978)
-
Method for synthesizing metronidazole under catalysis of solid acid
-
Paragraph 0029-0058, (2019/09/05)
The invention discloses a method for synthesizing metronidazole under catalysis of solid acid. The method comprises the following steps that 2-methyl-5-nitroimidazole as a raw material and the solid acid as a catalyst react with ethylene oxide to obtain the metronidazole, wherein a reaction system is filtered to recycle the solid acid; a filtrate is concentrated and evenly mixed by adding water, and the pH value is adjusted to be 2-3 by adding alkali; the 2-methyl-5-nitroimidazole is recycled by filtration, the pH value of the filtrate is adjusted to be 10 again by adding alkali, and then themetronidazole can be obtained. The synthesizing process route is simple, the production cost is low, and the solid acid catalyst is environmentally friendly and can be recycled.
Synthetic method for producing metronidazole raw medicinal material
-
Paragraph 0013-0014, (2018/07/30)
The invention discloses a synthetic method for producing a metronidazole raw medicinal material. The synthetic method comprises the following steps: (1) a synthetic reaction: putting reaction raw materials including 2.4 to 2.6 g of 2-methyl-5-nitroimidazole, 73 ml of absolute ethyl alcohol, 1.5 to 2 g of ethylene chlorohydrin and 4.5 to 5 g of K2CO3 into a reaction tank, raising the temperature to75 DEG C, preserving the temperature, controlling the temperature at 75 to 80 DEG C, reacting for 8 to 10 h, and stopping the reaction; and (2) carrying out immediate filtration in which a filter cake is byproduct potassium chloride, continuously evaporating a filter liquor to dryness, using methylene dichloride to dissolve at the temperature of 40 DEG C, cooling, separating out crystals, filtering, drying, and thus obtaining metronidazole. The method disclosed by the invention is mild in preparation condition and low in cost, and is applicable for industrial production.
Tritiated metronidazole and preparation method thereof
-
Paragraph 0044; 0045, (2017/08/28)
The invention belongs to the field of radioactive isotope labeling preparation, and particularly relates to tritiated metronidazole and a preparation method thereof. The preparation method includes: using 2-methyl-5-nitroimidazole as a raw material to react with N-iodosuccinimide to obtain 4-iodine-2-methyl-5nitroimidazole; under catalysis of palladium carbon, enabling 4-iodine-2-methyl-5nitroimidazole and tritium gas to be in tritium-halogen exchange to generate 4-3H-2-methyl-5-nitroimidazole; enabling 4-3H-2-methyl-5-nitroimidazole to react with ethylene oxide to obtain 4-3H-metronidazole. A synthetic product is purified through a prepared liquid phase to obtain4-3H-metronidazole with high specific activity (22.08Ci/g), high radiochemical purity (greater than or equal to 98%) and high chemical purity (greater than or equal to 98%). The tritiated metronidazole can be used as a radioactive tracer in studying absorption, distribution, metabolism and residue elimination of metronidazole in animal bodies.
Environment-friendly method for metronidazole synthesis
-
Paragraph 0040; 0041; 0047; 0048, (2016/10/07)
The invention discloses an environment-friendly method for metronidazole synthesis. Formic acid is replaced with acetic acid, alcohol is added for esterification, neutralization is performed for three times, sodium sulfate and ethylene glycol are recovered, a nitration product is effectively recovered from a metronidazole mother solution, derivatives such as acetic ester, anhydrous sodium sulfate and the like are obtained, by-products such as ethylene glycol and the like are chemical raw materials with wide applications, resources are recycled, the raw materials are greatly saved, the production cost is reduced, and the whole novel process adopts simple steps and is convenient to operate.