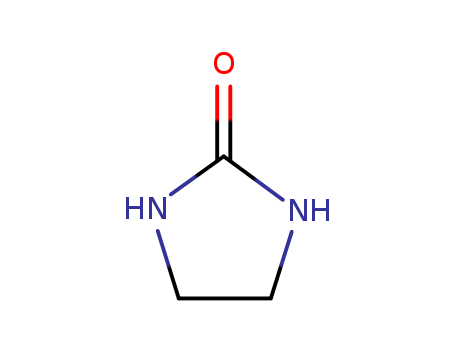
2-Imidazolidone literature
-
Naumov et al.
, (1973)
-
-
Sonoda,N. et al.
, p. 6344 (1971)
-
-
Staudinger,Niessen
, p. 75,87 (1955)
-
Synthesis of substituted ureas from urea and halohydrins
Magerramov,Abdinbekova,Kurbanova,Zamanova,Allakhverdiev
, p. 1667 - 1669 (2004)
Substituted ureas were prepared by reactions of 1,2-halohydrins with urea and were tested as antimicrobial additives to motor oils.
Kinetics and mechanism for CO2 scrambling in a N-carboxyimidazolidone analogue for N1-carboxybiotin
Lihs, Fiona J.,Caudle, M. Tyler
, p. 11334 - 11341 (2002)
The N-carboxyimidazolidone anion, 2-, was prepared as an analogue for N1-carboxybiotin, and the kinetics of its CO2-dependent chemistry were studied in polar aprotic media. The objective was to assess the viability of unimolecular CO2 elimination from N1-carboxybiotin as a microscopic step in biotin-dependent carboxyl transfer enzymes. The anionic 2- was prepared as its lithium salt by first deprotonating 2-imidazolidone with phenyllithium, followed by direct reaction with carbon dioxide. This procedure also permitted isolation of the 13C enriched derivative 2-{13C} by reaction with 13 CO2. Proton and 13C NMR and isotope-dependent FTIR measurements confirmed that carboxylation had occurred at the nitrogen atom of 2-imidazolidone to give 2-. Time-dependent FTIR spectroscopy showed that Li2 undergoes carboxyl exchange with free carbon dioxide, with kinetics indicative of rate-limiting unimolecular dissociation of the N-CO2 bond. Under these conditions, the weak Lewis acid Mg2+ catalyses the exchange of 2- with free CO2, which appears to be related to the ability of the metal ion to coordinate to 2-. Reaction of Li2 with carboxylic acids in DMSO results in acid-dependent decarboxylation of 2- with a rate that is dependent on the concentration of the acid and its pKa. A common mechanistic framework is presented for both Lewis acid catalyzed carboxyl exchange and acid-dependent decarboxylation that involves initial interaction at the carbonyl oxygen and which has the effect of polarizing the nitrogen lone pair toward the imidazolidone ring rather than the carboxyl group. Lewis acid interaction with the carbonyl oxygen thus weakens the N-CO2- bond and functions as a trigger for dissociation of CO2. In the context of biotin-dependent enzymes, this suggests a means by which the kinetically stable N1-carboxybiotin cofactor intermediate might be triggered for dissociation of CO2.
-
Marshall,Singh
, p. 1316,1317, 1319 (1977)
-
ALKALINE HYDROLYSIS OF N-NITROSO-2-IMIDAZOLIDONE
Castro, Albino,Leis, J. Ramon,Pena, M. Elena,Tato, Jose Vazquez
, p. 117 - 122 (1986)
The hydrolysis of N-nitroso-2-imidazolidone has been studied kinetically between pH 8.3 and 12.6.This nitroso compound has an acid-base equilibrium whose constant has been determined spectrophotometrically (pKa 11.45).Only the acid form is reactive.At pH < 10 hydrolysis is of order less than one with respect to OH- and is subject to general base catalysis.These results are interpreted in terms of a mechanism involving an initial steady-state hydrate whose decomposition by base leads to the final products.At pH > 10 reaction paths of orders one and two in OH- appear.The second-order term reflects general base catalysis superimposed on a first-order term in OH- (the bases dimethylamine, sarcosine, piperidine, and HPO42- have been used).The results are interpreted by an initial OH- attack on the carbonyl group of the nitroso compound to give an intermediate which in the rate-controlling step reacts with bases, among them water (which explains the first-order term with respect to OH-).The low value of the Broensted relation (β ca.O) and the fact that the intermediate possesses no proton yielding a low pKa value suggest that there is inverse classical general base catalysis.
Ethylenethiourea S-Oxidation Products: Preparation, Degradation, and Reaction with Proteins
James, Joyce P.,Quistad, Gary B.,Casida, John E.
, p. 2530 - 2535 (1995)
Ethylenethiourea (ETU) is carcinogenic degradation product of major ethylene bis(dithiocarbamate) fungicides with biological activity attributed to poorly characterized oxidation products.The reaction of ETU with H2O2 was examined in aqueous medium at pH percent, 7, and 9 by 1H NMR spectroscopy giving five principal products: sequential formation of sulfenic, suflinic, and sulfonic acids as well as imidazoline and ethyleneurea.Maximum yields with 2 equiv of H2O2 at optimal pH were 10, 71, 5, 53, and 100percent, respectively.Oxidation proceeds mainly through the sulfinic acid to imidazoline in acidic medium and the S-oxide to ethyleneurea in basic medium. 1H NMR of urine from mice treated with ETU revealed ETU and imidazoline (no ethyleneurea or S-oxidation products).Albumin was radiolabeled in ca.17percent yield by a 10-fold excess of <14C>ETU/H2O2 oxidation products (primatily the sulfenic and sulfinic acids), and such protein modification aould be prevented by glutathione.Keywords: Ethylenethiourea; ethylenethiourea S-oxidation products; hydrogen peroxide; imidazoline; protein adducts; sulfenic acid
-
Schweitzer
, p. 471,472 (1950)
-
-
Zav'yalov et al.
, (1971)
-
CO2-Fixation on Aliphatic α,ω-Diamines to Form Cyclic Ureas, Catalyzed by Ceria Nanoparticles that were Obtained by Templating with Alginate
Primo, Ana,Aguado, Eric,Garcia, Hermenegildo
, p. 1020 - 1023 (2013)
Ceria nanoparticles (average particle size: 8nm) have been obtained by the calcination of alginate aerogel beads that were precipitated from aqueous solutions of (NH4)2Ce(NO3)6. These nanoparticles were considerably more active as a catalyst for CO2-insertion into aliphatic α,ω-diamines than the analogous commercial CeO2 with larger particle size (40nm). CeO2 that was obtained by templating with the natural alginate biopolymer afforded the cyclic urea of ethylenediamine in EtOH solvent at 160°C in 37% yield. This yield is remarkable for a process that involves CO2 as a feedstock. Other α,ω-diamines, such as diethylenetriamine, N,N′-dimethylethylenediamine, N-(2-aminoethyl)acetamide, and 1,4-diaminobutane, also formed their corresponding cyclic ureas in 4-36% yield. The catalyst lost activity upon reuse, thereby leading to severe deactivation that was only partially recovered by washing with aqueous acidic solutions.
Structure and conformational analysis of a macrocyclic ligand: [24, 26-DIOXO-3,6,14,17-tetraazapentacyclo (21.0.11,19.1 3,6.18,12.114,17)Hexacosan-1(23),8(25),9,11,19, 21-hexaene]
Sony, S.M. Malathy,Kuppayee,Ponnuswamy,Murali,Rajakumar
, p. 85 - 92 (2003)
24,26-Dioxo-3,6,14,17-tetraazapentacyclo((21.0.11,19.1 3,6.18,12.114,17)hexacosan-1(23),8(25),9,11,19, 21-hexaene, C22H24N4O2 FW=376.45, monoclinic, P21/n,a = 11.144(2) A, b = 6.395(1) A, c = 13.562(3) A, β = 95.81(1)°, V= 961.5(3) A3, Z =2, Dcol = 1.300 Mg/m3, μ = 0.685 mm-1, F(000)=400, λ (CuKα) = 1.5418 A final R1 and wR2 are 0.0409 and 0.1547, respectively. The macro ligand consists of two phenyl rings and two five-membered rings forming the walls of the central cavity which has roughly a square cross-section. The orientation of the phenyl ring is antiperiplanar and approximately perpendicular to the diazacyclopentanone ring which adopts envelope conformation. The molecules are stabilized by C-H ... O and C-H ... π types of intermolecular interactions in addition to van der Waals forces.
Efficient and Green Preparation of 2-Imidazolidinone using Sulfamic Acid as Acidic Catalyst
Liu, Jianshe,Xie, Yaqiang,Ye, Liyi,Yan, Huidong,Tu, Song
, p. 453 - 456 (2014)
-
-
Klut
, p. 677 (1902)
-
-
McKay et al.
, p. 3205 (1950)
-
ENHANCED REACTIVITY OF 1-CARBOXY-2-ETHOXY-2-IMIDAZOLINE, A BIOTIN MODEL, IN DECARBOXYLATION
Kondo, Hiroki,Miura, Katsuhito,Sunamoto, Junzo
, p. 659 - 662 (1982)
Summary: 1-Carboxy-2-ethoxy-2-imidazoline (1) decarboxylates five times as fast as 1-carboxy-2-imidazolidinone (2) in an aqueous alkaline medium.
SBA-15 Supported Dendritic ILs as a Green Catalysts for Synthesis of 2-Imidazolidinone from Ethylenediamine and Carbon Dioxide
Liu, Jinghan,Ma, Jianjun,Miao, Penghua,Min, Qingwang,Qi, Meijuan,Shamsa, Farzaneh
, (2021/07/26)
In this work, a simple and facile approach is conducted for preparing many new SBA-15 supported dendritic imidazolium ILs heterogeneous catalysts SBA-15/IL(1–3) having high ionic density from SBA-15. SBA-15/IL(3) as a green heterogeneous catalyst can be used for synthesis of 2-imidazolidinone from ethylenediamine and carbon dioxide and considering solvent-free condition. SBA-15/IL(3) showed to have the highest catalytic activity besides a positive dendritic influence on the yields of the synthesis of 2-imidazolidinone in the presence of CO2 is seen because of existing the high-density peripheral zwitterionic ionic liquid functional groups on the biobased SBA-15/IL(3) catalyst surfaces. Graphical Abstract: [Figure not available: see fulltext.]
Synthesis method of heterocyclic compound containing bis (trimethylsilyl)
-
Paragraph 0029; 0034-0035; 0038; 0043-0044; 0065; 0067; ..., (2021/09/29)
The method comprises the following steps: first adopting bis (trichloromethyl) carbonate, ethylenediamine, an aqueous sodium hydroxide solution, chloroform and a supported catalyst to obtain 2 - imidazolidinone. Or, ethylenediamine, ethanol, distilled water, a supported catalyst, carbon disulfide and hydrochloric acid are prepared to obtain ethylene thiourea. The trimethylchlorosilane is dissolved in an organic solvent, ammonia gas is introduced, and heated to reflux until no white precipitate is generated and filtered to obtain the filtrate. The raw material 2 - imidazolidinone or ethylidene thiourea is then dissolved to obtain the raw material solution, ammonium sulfate is added, and the filtrate is added dropwise, heated and refluxed 5 - 6h, cooled to room temperature, filtered, and the product is obtained. The molar ratio of the raw material to the trimethyl chlorosilane is 1: (2.1 - 2.5), the quality of ammonium sulfate is 1.5 - 2% of the raw material. The preparation method is simple and high in yield.
METHOD OF PREPARING UREA USING AMINE COMPOUND AND CARBON DIOXIDE
-
Page/Page column 10-15, (2020/11/14)
Disclosed is a production method of urea using an amine compound and carbon dioxide. The production method of urea includes a step of producing urea by using the amine compound and a 2-pyrrolidone derivative as a solvent and reacting with the carbon dioxide, thereby producing high yield cyclic urea under mild reaction conditions and no catalyst conditions.
Rational design of bifunctional catalyst from KF and ZnO combination on alumina for cyclic urea synthesis from CO2 and diamine
John, Crowny,Kulal, Nagendra,Shanbhag, Ganapati V.
, (2020/04/22)
This study is mainly focused on the design of stable, active and selective catalyst for direct synthesis of 2-imidazolidinone (cyclic urea) from ethylenediamine and CO2. Based on the rationale for the catalyst properties needed for this reaction, KF, ZnO and Al2O3 combination was selected to design the catalyst. ZnO/KF/Al2O3 catalyst was prepared by stepwise wet-impregnation followed by the removal of physisorbed KF from the surface. High product yield could be achieved by tuning acid-base sites by varying the composition and calcination temperature. The catalysts were characterized by various techniques like XRD, N2-sorption, NH3-TPD, CO2-TPD, TEM, XPS and FT-IR measurements. It is shown that acidic and basic properties of the solvent can influence the activity and product selectivity for this reaction. Under optimized condition; 180 °C, 10 bar and 10 wt.% catalyst in batch mode, 96.3 % conversion and 89.6 % selectivity towards the 2-imidazolidinone were achieved.