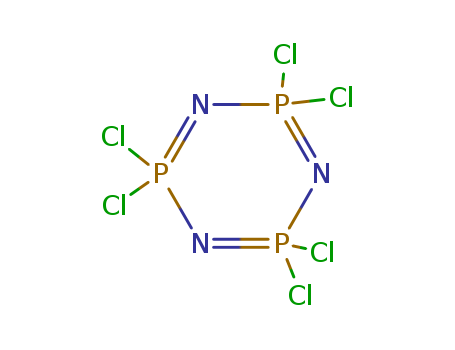
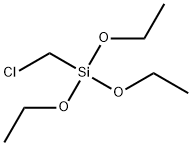
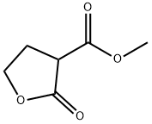
Phosphonitrilic chloride trimer literature
Purification method of hexaphenoxy cyclotriphosphazene
-
Paragraph 0017-0019, (2020/10/30)
The invention discloses a purification method of hexaphenoxy cyclotriphosphazene. The method comprises the following steps: firstly, adding a 5-10% sodium hydroxide solution into a hexaphenoxy cyclotriphosphazene synthesis liquid, stirring for 10 to 30 minutes at room temperature, standing, separating out a water phase, adding water, stirring at room temperature for 10-30 minutes, standing, separating out the water phase, adding a 5-10% hydrochloric acid solution, stirring at room temperature for 10-30 minutes, standing, separating out the water phase, adding water, stirring at room temperature for 10-30 minutes, standing, and separating out the water phase; finally steaming out chlorobenzene in the washed oil phase to obtain a hexaphenoxy cyclotriphosphazene crude product; and heating anddissolving the crude product with absolute ethyl alcohol, cooling to -10-0 DEG C, crystallizing for 4 to 8 hours, filtering, washing a filter cake with absolute ethyl alcohol twice, and drying at 90to 100 DEG C until the weight is constant so as to obtain the hexaphenoxy cyclotriphosphazene. According to the purification method disclosed by the invention, the yield of the hexaphenoxy cyclotriphosphazene is remarkably improved.
Phosphazene compound, composite containing phosphazene compound, flame retardant containing composite and application
-
Paragraph 0221; 0224-0227, (2019/01/23)
The invention relates to a phosphazene compound, a composite containing the phosphazene compound, a flame retardant containing the composite and application. The crystal form of the cyclotriphosphazene derivative flame retardant synthesized by using the method is most stable, and the cyclotriphosphazene derivative flame retardant is high in heat stability and high in flame retardant efficiency; moreover, when the crystal type cyclotriphosphazene derivative flame retardant synthesized by using the method is added into a material, such as engineering plastics, general-purpose plastics, a lithiumion battery electrolyte, a flame retardant fabric and flame retardant paper, a high flame retardant performance is achieved, the crystal form is stable, the flame retardant is resistant to drip, onlyan extremely small adding amount is needed, the flame retardant performance of the material can reach the V-0 standards, and other properties of the material are little affected.
ALLYL-PHENOXY-CYCLOPHOSPHAZENE COMPOUND, AND PRODUCTION METHOD THEREFOR
-
Paragraph 0217, (2018/06/15)
An object of the present invention is to provide a mixture of cyclophosphazenes suitably substituted with phenoxy having a polymerizable functional group, such as allyl, on the phenyl ring and a production method for the mixture. The invention relates to a mixture of cyclophosphazene compounds that each contain a plurality of constituent units linked to each other, each constituent unit being represented by formula (I): wherein R1 and R2 are identical or different and represent C1-4 alkyl or the like, the mixture containing cyclophosphazene compounds in which 3, 4, and 5 constituent units represented by formula (I) are linked, wherein the cyclophosphazene compound containing 3 linked constituent units is cyclophosphazene compound (I-A) with a specific structure, compound (I-A) includes cyclophosphazene compounds (I-A2) and (I-A3) having a specific structure, and compounds (I-A2) and (I-A3) are present in an amount of 80 wt % or more in total in cyclophosphazene compound (I-A).
Cyclotriphosphazene compound, preparing method thereof, electrolyte for lithium secodary battery, and lithium secodary battery including the same
-
Paragraph 0128-0134, (2017/08/19)
A cyclotriphosphazene compound in which at least one fluorine is substituted with a group represented by formula 1 below, a preparing method thereof, an electrolyte for a lithium secondary battery including the cyclotriphosphazene compound, and a lithium secondary battery including the electrolyte are provided. Formula 1 is *A-B-CN, where A is a heteroatom having an unshared electron pair, * represents a combined site of the fluorinated cyclotriphosphazene compound with phosphor (P), and B is a substituted or unsubstituted alkylene group having 1 to 5 carbon atoms.